|
 |
- Search
| Phys Act Nutr > Volume 27(3); 2023 > Article |
|
Abstract
[Purpose]
[Methods]
[Results]
[Conclusion]
Acknowledgments
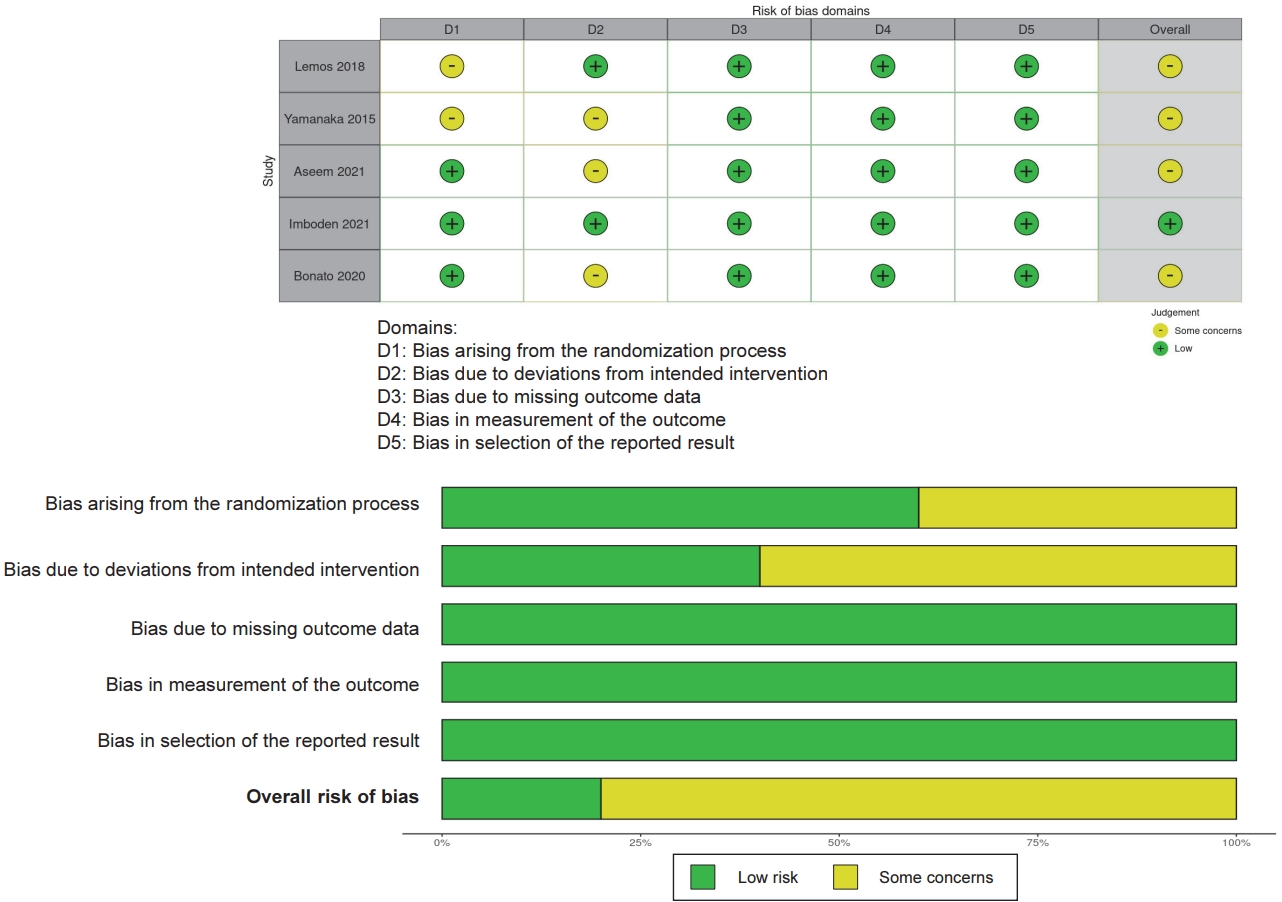
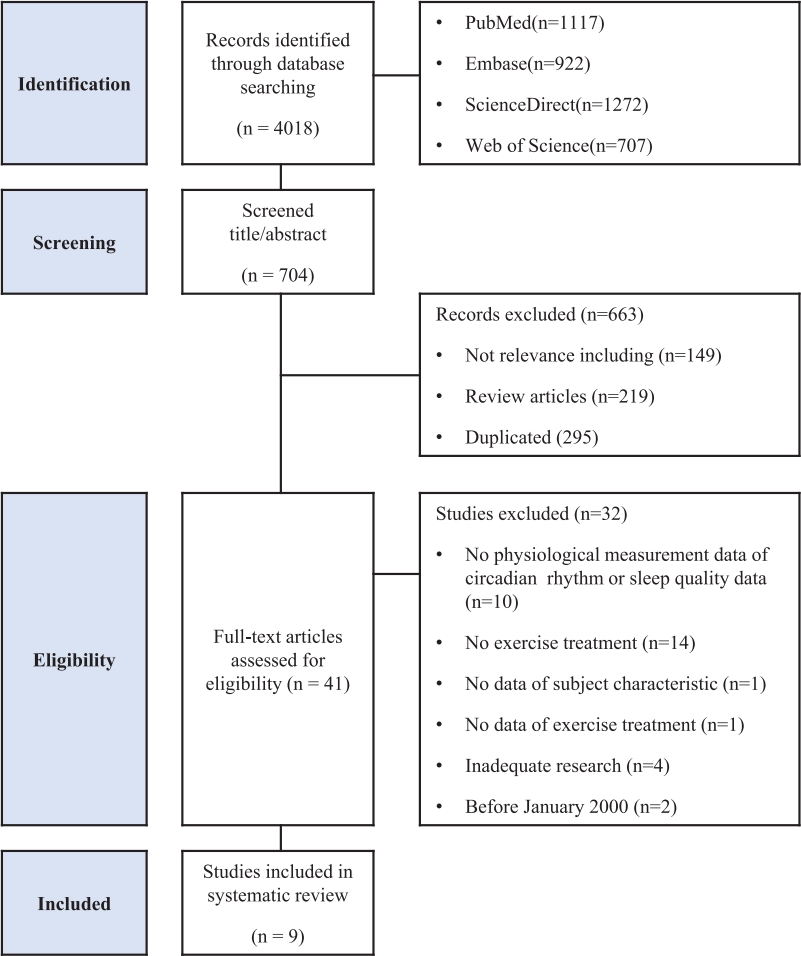
Figure┬Ā1.
Table┬Ā1.
| Studies |
Population |
Exercise, Training |
Comparison |
Outcomes of circadian rhythm |
Outcomes of sleep quality |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) | Sample size (male) | Health status | Type | Intensity | Time | Timing | Frequency/Duration | Circadian rhythm measurement | Sampling timing | Relevant findings | Sleep quality measurement | Relevant findings | ||
| Lemos et al., 2018 | 26 ┬▒ 3 | 40 (40) | Generally healthy | Normoxia Treadmill | 50% VO2max | 60 min | Morning 11:00 | Acute | Normoxia no exercise | Plasma melatonin | 19:00 on exercise day, | No significantly different melatonin concentration Ōåö | PSG | Improved sleep onset latency, total sleep time |
| Ō¢Ā Sleep onset latency (min) Ōåō | ||||||||||||||
| Ō¢Ā Total Sleep Time (min) Ōåæ | ||||||||||||||
| 4500m Hypoxia Treadmill | 4500m Hypoxia no exercise | 7:30 next exercise day | Significantly higher melatonin concentration at 19:00 on exercise day Ōåæ | Improved sleep onset latency, total sleep time, and sleep efficiency | ||||||||||
| Ō¢Ā Sleep onset latency (min) Ōåō | ||||||||||||||
| Ō¢Ā Total Sleep Time (min) Ōåæ | ||||||||||||||
| Ō¢Ā Sleep efficiency (%) Ōåæ | ||||||||||||||
| Yamanaka et al., 2015 | 22 ┬▒ 2 | 22 (22) | Generally healthy | Bicycle ergometer | 65%-75% HRmax | 2 hours | Morning 10:00 - 12:00 | consecutive 4 days (day 3-6) | Pre exercise (day1) | Plasma melatonin rhythms b) | day 6 | Increased melatonin onset Ōåæ, Increased melatonin peak Ōåæ, No significant different offset Ōåö b) | PSG | No significantly different sleep quality |
| Onset phase delayed | Ō¢Ā Sleep latency (min) Ōåö | |||||||||||||
| Peak phase delayed | Ō¢Ā Sleep efficiency (%) Ōåö | |||||||||||||
| Core body temperature | day 6 14 - 8 Zeitgeber time (h) | No significantly different core body temperature Ōåö | Ō¢Ā SWS (min) Ōåö | |||||||||||
| Ō¢Ā REM sleep (min) Ōåö | ||||||||||||||
| Evening 17:00-19:00 | Plasma melatonin rhythmsb) | day 6 | Increased melatonin onset Ōåæ, Increased melatonin peak Ōåæ, Increased melatonin offset Ōåæb) | PSG | Decreased REM sleep (min) Ōåō | |||||||||
| Onset phase delayed | Ō¢Ā Sleep latency (min) Ōåö | |||||||||||||
| Peak phase delayed | Ō¢Ā Sleep efficiency (%) Ōåö | |||||||||||||
| Offset phase delayed | Ō¢Ā SWS (min) Ōåö | |||||||||||||
| Core body temperature | day 6 14 - 8 Zeitgeber time (h) | Increased core body temperatureŌåæ | Ō¢Ā REM (min) Ōåō | |||||||||||
| Passos et al., 2014 | 45 ┬▒ 9 | 21 (5) | Chronic primary insomnia | Treadmill | Ventilatory thresholds 1 | 50 min | 10:00 or 18:00 | 3 days/week | Pre exercise | Plasma cortisol | 10:00 - 11:00 | Decreased cortisol concentration Ōåō | PSG | Improved sleep quality |
| Ō¢Ā total sleep time Ōåæ | ||||||||||||||
| Ō¢Ā Wake after sleep onset (min) Ōåō | ||||||||||||||
| Moderate intensity | 4 months | PSQI | Ō¢Ā Sleep efficiency (%) Ōåæ | |||||||||||
| Ō¢Ā REM sleep (%) Ōåæ | ||||||||||||||
| Ō¢Ā PSQI Score Ōåō c) | ||||||||||||||
| Flausino et al., 2012 | 27 ┬▒ 4 | 17 (17) | Generally healthy good sleepers | Treadmill | Ventilatory thresholds 1 | 30 min | Evening 20:00-20:30 | Acute (non- consecutive days) | Pre exercise (day 1) | Core body temperature | 0 - 300 min after exercise | No significantly different core body temperature Ōåö | PSG | Improved sleep quality |
| Ō¢Ā Wake after sleep onset (min) Ōåō | ||||||||||||||
| Moderate intensity | Ō¢Ā Sleep efficiency (%) Ōåæ | |||||||||||||
| Ō¢Ā stage 1 sleep (%) Ōåō | ||||||||||||||
| Ventilatory thresholds 1 | 60min | Increased core body temperature after exercise Ōåæ and returned to baseline levels 120 min later | Improved sleep quality | |||||||||||
| Ō¢Ā Wake after sleep onset (min) Ōåō | ||||||||||||||
| Moderate intensity | Ō¢Ā Sleep efficiency (%) Ōåæ | |||||||||||||
| VO2peak = 46 ┬▒ 7 | 50% above Ventilatory thresholds 1 | 30 min | No significantly different core body temperature Ōåö | Improved sleep quality | ||||||||||
| Ō¢Ā Wake after sleep onset (min) Ōåō | ||||||||||||||
| Ō¢Ā Sleep efficiency (%) Ōåæ | ||||||||||||||
| Ō¢Ā stage 1 sleep (%) Ōåō | ||||||||||||||
| 50% above Ventilatory thresholds 1 | 60 min | Increased core body temperature after exercise Ōåæ and returned to baseline levels 30 min later | Improved sleep quality | |||||||||||
| Ō¢Ā Wake after sleep onset (min) Ōåō | ||||||||||||||
| Ō¢Ā Sleep efficiency (%) Ōåæ | ||||||||||||||
| Ō¢Ā Stage 1 sleep (%) Ōåō | ||||||||||||||
| Imboden et al., 2021 | 40 ┬▒ 11 | 42 (22) | Depression | Indoor bicycles | 60-75% HRmax | 40 - 50 min | Daytime 16:00 - 18:00 | 3 times/week | Active control (Stretching) | Salivary cortisol CAR | immediately after waking up, 10, 20, 30 min later | No significantly different CAR Ōåö | PSG | No significantly different sleep quality |
| Ō¢Ā PSG Ōåö | ||||||||||||||
| Ō¢Ā PSQI Score Ōåö c) | ||||||||||||||
| Indoor bicycles | 60-75% HRmax | Pre exercise | Decreased CAR Ōåō | Improved sleep quality | ||||||||||
| PSQI | Ō¢Ā PSG Ōåö | |||||||||||||
| 6 weeks | Ō¢Ā PSQI Score Ōåō c) | |||||||||||||
| Active control (Stretching) | Pre exercise | Decreased CAR Ōåō | Improved sleep quality | |||||||||||
| Ō¢Ā PSG Ōåö | ||||||||||||||
| Ō¢Ā PSQI Score Ōåō c) | ||||||||||||||
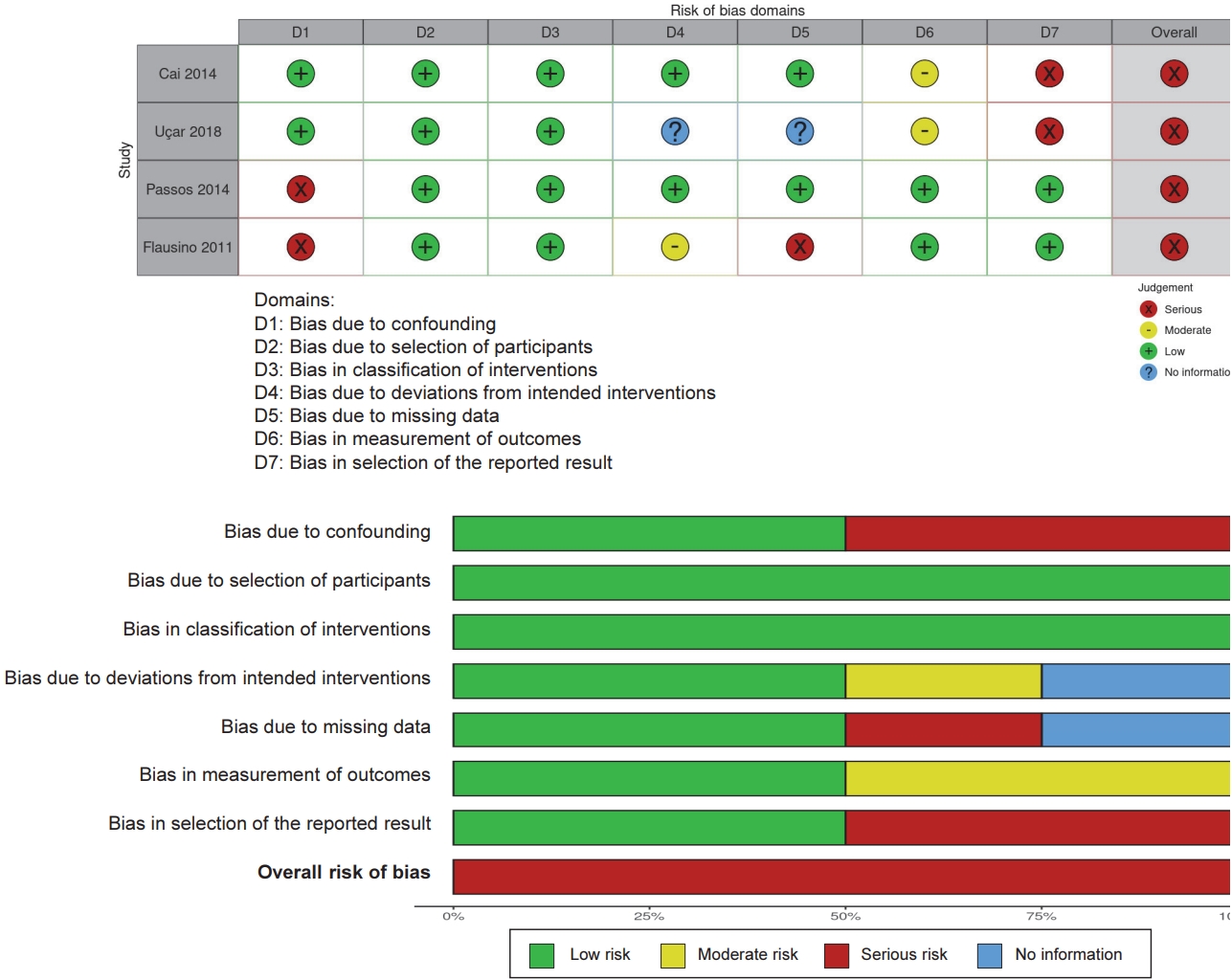
| Cai et al., 2014 | 58 ┬▒ 1 | 10 (0) | sleep disturbance postmenopausal sedentary | Groupbased step aerobic exercise | 75-85% HRR | 40-45 min | Morning 8:30, 10:00 | 3 times/week | Pre exercise | Blood melatonin | before and after the 10 weeks program (between 07:30 and 09:30) | Increased melatonin concentrationŌåæ | PSQI (Chinese version) | Improved sleep quality |
| 10 weeks | Ō¢Ā PSQI Score Ōåōc) | |||||||||||||
| Aseem et al., 2021 | 22 ┬▒ 3 | 14 (14) | Sleep disturbance (PSQI>5) | Treadmill | 85% HRmax | 60 min | Forenoon (Morning) | 3 times/week | Pre exercise | Serum melatonin | Before sleep | No significant different melatonin concentration Ōåö | PSG | Improved sleep quality |
| 12 weeks | Serum cortisol | after waking up | Decreased cortisol concentration Ōåō | Ō¢Ā N1 sleep (%) Ōåō | ||||||||||
| Ō¢Ā N3 sleep (%) Ōåæ | ||||||||||||||
| U├¦ar et al., 2018 | 22 (20-24)a) | 20 (20) | Generally healthy | Football match | Hard (Borg scale) | 90 min | Evening 21:30 | acute | Pre exercise | Salivary cortisol CAR | after awakening on the next day | No significantly different CAR Ōåö | PSQI | No significantly different sleep quality |
| Ō¢Ā PSQI Score Ōåö c) | ||||||||||||||
| Bonato et al., 2020 | 23 ┬▒ 6 | 15 (15) | Non-professional soccer players | HIIT 4 bouts of 4min (4 ├Ś 4) at 90- 95% HRpeak with 3 min of active recovery at 50- 60% HRpeak. | Evening 20:00 | acute | Small-sided games group (4 ├Ś 4) at 90-95% HRpeak with 3 min of active recovery | Salivary cortisol CAR | post exercise, CAR: 30 minutes after morning awaking | Significantly higher cortisol concentrations at post exercise Ōåæ | Actigraphy | Decreased sleep quality | ||
| Ō¢Ā Actual sleep time (%) Ōåō | ||||||||||||||
| Ō¢Ā Sleep efficiency (%) Ōåō | ||||||||||||||
| Significantly higher CAR Ōåæ | Ō¢Ā Immobility time Ōåō | |||||||||||||
| Ō¢Ā Moving time Ōåæ | ||||||||||||||
Abbreviation. HIIT: High intensity interval training, CAR: Cortisol Awakening Response; AUC: Area under curve, PSQI: Pittsburgh Sleep Quality Index, PSG: Polysomnography, SWS: Slow-wave sleep, REM: Rapid eye movement, HRR: Heart rate reserve.
Ōåö: no significantly difference (p > 0.05)
Ōåæ: significantly increased or higher than comparison (p < 0.05)
Ōåō: significantly decreased or lower than comparison (p < 0.05)
b) The onset and offset phases of nocturnal melatonin rise were defined as the time at which the horizontal line of 20% amplitude crossed the ascending and descending portions. The peak phase was the midpoint between the onset and offset phases. The phase shift was calculated by comparing the differences in the phases (onset, peak, offset) of melatonin rhythm on before and after exercise (35).
REFERENCES
-
METRICS

-
- 0 Crossref
- Scopus
- 1,296 View
- 68 Download
- Related articles in Phys Act Nutr
-
Articles : The Effects of Exercise Intensity on Thermophysiological Responses2003 March;7(1)