 |
 |
- Search
| Phys Act Nutr > Volume 27(4); 2023 > Article |
|
Abstract
[Purpose]
One of the urgent research projects in exercise science should focus on sports supplements for obese people who lack exercise and physical activity. In this study, we explored the efficacy in non-alcoholic fatty liver disease (NAFLD) mice models using a Korean herbal medicine Erigeron breviscapus (EB).
[Methods]
Gene ontology analyses of active compounds in EB were performed using the Traditional Chinese Medicine Database and Analysis Platform (TCMSP) and Cytoscape program, respectively. PA-induced acid (PA) induced-lipid droplets in HepG2 cells were analyzed using a 3D-hologram. To analyze the fat-suppressing efficacy of EB in animal experiments, NAFLD was induced through a 24-week high-fat diet. Subsequently, the same diet was continued for an additional 8 weeks, with concurrent co-administration of drugs for efficacy analysis. In the 8-week experiment, mice were administered saline alone, metformin (17 mg/kg/day), or EB (26 mg/kg/day). The mice were sacrificed and the liver tissue was isolated. The liver tissues were stained with H&E and specific antibodies such as sterol regulatory element binding protein 1 (SREBP-1) and peroxisome proliferator-activated receptor- ╬│ (PPAR-╬│).
[Results]
Seventeen EB-active compounds were identified by whole-body analysis. EB downregulated lipid droplets in PA-treated HepG2 cells. EB regulates lipid accumulation in liver tissue of HFD-fed NAFLD mice Metformin and EB significantly reduced the expression of SREBP-1 and PPAR-╬│ in liver tissue.
The prevalence of obesity is intricately linked to contemporary lifestyle habits, characterized by a Western diet, sedentary living, and a decline in physical activity. These factors contribute significantly to the rise in metabolic disorders, including atherosclerosis and non-alcoholic fatty liver disease (NAFLD) [1-3]. According to a review paper by Philipp Kasper et al., the global prevalences of NAFLD and nonalcoholic steatohepatitis (NASH) are approximately 25% and 5% [4]. In addition, NAFLD manifests as the accumulation of fat in the liver without alcohol consumption. Nonetheless, it evolves into a grave condition progressing through stages such as NASH, liver fibrosis, cirrhosis, and hepatocellular carcinoma, culminating in fatal outcomes [5]. The factors influencing NAFLD development remain unclear. This liver ailment arises from fat accumulation even without alcohol consumption, yet its precise cause is uncertain and closely linked to obesity. Unfortunately, the high mortality rate associated with NAFLD is attributed to the absence of distinctive symptoms recognizable by patients [6,7]. Medically recommended, 10% weight loss is known to facilitate the resolution of nearly universal NAFLD and improvement of NASH [8]. Exercise emerges as a powerful treatment, reducing the mortality rate of various diseases, and has a particularly strong impact on weight loss [9]. However, specialized training is imperative to enhance an individualŌĆÖs physical capacity in intense exercise programs designed to address NAFLD. Therefore, there is an urgent need to develop effective exercise methods and supplements to improve weight loss and prevent NAFLD.
Carbohydrates are converted to fat in the liver and stored, and the continuous synthesis and excessive storage of fat can cause diseases such as NAFLD [10]. During lipogenesis, sterol regulatory element-binding proteins (SREBP-1) are the major transcription factors that regulate cholesterol homeostasis and lipid metabolism [11]. Moreover, the progression of NAFLD is intricately governed by peroxisome proliferator-activated receptor- ╬│ (PPAR-╬│), which regulates adipogenesis and lipid metabolism [12]. Consequently, assessing the expression levels of SREBP-1 and PPAR-╬│ serves as a crucial indicator for monitoring the regulation of lipid accumulation. Furthermore, one of the NAFLD treatment targets is suggested that the regulation of SREBP-1 and PPAR-╬│.
Natural nanoparticles (NPs) have long been used in food and medicine. In particular, NPs have been reported to have various biological and physiological functions such as anti-inflammatory, antioxidant, and anti-cancer effects [13]. Erigeron breviscapus (EB) is a medicinal herb containing large amounts of flavonoids [14]. However, the role of EB in the regulation of lipogenesis has not yet been studied. Here, we sought to find the fundamental data on how EB can help treat NAFLD and should report meaningful results on SREBP-1 and PPAR-╬│ regulation.
To analyze the components of EB and the target gene network, we used the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) and Cytoscape. EB characteristics analyze of EB was analyzed as previously described [15]. The TCMSP (http://ibts.hkbu.edu.hk/LSP/tcmsp.php) was used to identify the active components of EB. As recommended by TCMSP, bioactive substances were analyzed based on their pharmacokinetic properties (absorption, distribution, metabolism, and excretion (ADME)), including oral bioavailability (OB), Caco-2 permeability (Caco-2) for intestinal epithelial permeability, and drug-likeness (DL). For further analysis, the active compounds with OB Ōēź 30%, Caco-2 Ōēź -0.4, and DL Ōēź 0.18, were selected as candidate compounds. The active components of EB exert their biological functions through specific targets. After directly obtaining the potential targets of the core active ingredients in EB from the TCMSP database, the protein names of all target genes were converted into corresponding gene symbols using the UniProt database. Gene ontology (GO) analysis was performed to identify the biological processes related to the collected genes using the DAVID 6.8 Gene Functional Classification Tool (https://david.ncifcrf.gov/). The p-values from the enrichment results underwent correction through the Benjamini-Hochberg method, with significance set at p < 0.01. To decipher the mechanisms of Ellagic Acid (EB) based on active ingredients and target networks, we utilized Cystoscope visualization software version 3.7.2 to construct compound-target networks (https://cytoscape.org/). The selected candidate ingredients and targets were input into the software and the network was analyzed. The relationships between various active EB ingredients and target genes, as well as the biological metabolic processes related to exercise metabolism, were selected, and a process-target network (PT network) was subsequently constructed.
Cells were cultured as described previously [16]. Briefly, HepG2 cells ( human hepatoma cell line) were obtained from the American Type Culture Collection (Rockville, MD, USA). The cells were maintained at 37 ┬░C and 5% CO2 in DulbeccoŌĆÖs modified EagleŌĆÖs medium containing 200 mg/L of l-glutamine (Hyclone, Logan, UT, USA), 10% (v/v) heat-inactivated fetal bovine serum (Invitrogen, Carlsbad, CA, USA), 100 U/mL penicillin (Invitrogen), and 100 ╬╝g/mL of streptomycin (Invitrogen).
HepG2 cells (1├Ś104 cells/well) were seeded in 96-well plates and incubated for 24 h. The cells were treated with diverse concentration of EB (10, 100, 1000 and 10000 ┬Ąg/mL) for 24 h. HepG2 cells were treated with WST-1 reagent for 2 h, and the cells were measured using a microplate reader at 450 nm.
Cells were cultured as described previously [16]. Brief, HepG2 cells (1├Ś105 cells/well) were seeded in 60-mm Tomodishes and treated with 500 ┬Ąg/mL EB at 36┬░C for 24 h. 3D holotomography images of live cells were obtained using an HT-1 microscope (magnification, ├Ś600; Tomocube, Inc.). Cell structures were scanned using a digital micromirror and reconstructed using a 3D refractive-index tomogram. The masses of the cytoplasm and lipid droplets were analyzed based on densities of 0.135 and 0.178 g/ml, respectively.
Six-week-old C57BL/6 mice (6 weeks) were purchased from Orient Bio (Gyeonggi-do, Korea). Animal care was performed as previously described [17]. All procedures involving animals were approved by the Institutional Animal Care and Use Committee of Semyung University (approval number 20-10-02), and the experimental protocols complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All the experiments were conducted over 32 weeks. A them high-fat diet was provided for 24 weeks to create animal models, and the untreated group was fed a standard diet. The mice were divided into three groups as follows: HF group, high-fat diet for 8 weeks; ME group, high-fat diet with the positive control (orally administered 17 mg of metformin/kg/day) for 8week, EB group, high-fat diet with natural products (orally administered 26 mg of Erigeron breviscapus /kg/day) for 8 weeks. The untreated (UN) group received the AIN 93G stock diet for 32 weeks, while the HF, ME, and EB groups were provided with a high-fat diet comprising 60% fat, 20% carbohydrates, and 20% protein (5.21 Kcal) based on lard. Subsequently, all mice underwent anesthesia via an intraperitoneal injection of sodium pentobarbital (25 ╬╝g/10 g body weight).
Immunohistochemistry was performed as previously described [18]. Briefly, the mice were perfused and fixed in 10% formalin, and then the liver specimens were prepared according to the routine procedures for cryosection. The sections were stained with hematoxylin and eosin. One set of specimens underwent a 3-minute treatment with 0.3% hydrogen peroxide to inhibit endogenous peroxidase activity, followed by a 1 h blocking step using 5% goat serum. The remaining specimens were then incubated with different primary antibodies in 3% goat serum at 4 ┬░C for 72 h. Antibodies to SREBP-1 and PPAR-╬│ were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The tissues were subsequently washed three times using phosphate-buffered saline, and the Vectastain ABC Kit was used to induce avidin-biotin complex interactions, according to the manufacturerŌĆÖs instructions. Immunostaining analysis was performed using a substrate solution of 0.05% DAB, and the slides were counterstained with hematoxylin. All the sections were evaluated under a light microscope (BX50; Olympus Co., Ltd., Hachioji-shi, Tokyo, Japan) at ├Ś 200 magnification.
Statistical data are expressed as mean ┬▒ standard error, based on at least three independent experiments. All data were analyzed using GraphPad Prism software (Prism 4.00 Windows, GraphPad, San Diego, CA, USA), based on StudentŌĆÖs t-test for intergroup differences and one-way analysis of variance with TukeyŌĆÖs test for multiple comparisons. Statistical significance was set at P < 0.05. significant.
To identify the active compounds in EB, we used the TCMSP database. Table 1 lists 17 bioactive EB compounds. Next, we identified the active molecule-related genes and analyzed the gene network. A total of 111 genes were associated with 17 active compounds. As shown in Figure 1, the gene networks were analyzed using Cytoscape. A total of 111 genes were associated with the top 31 gene ontologies.
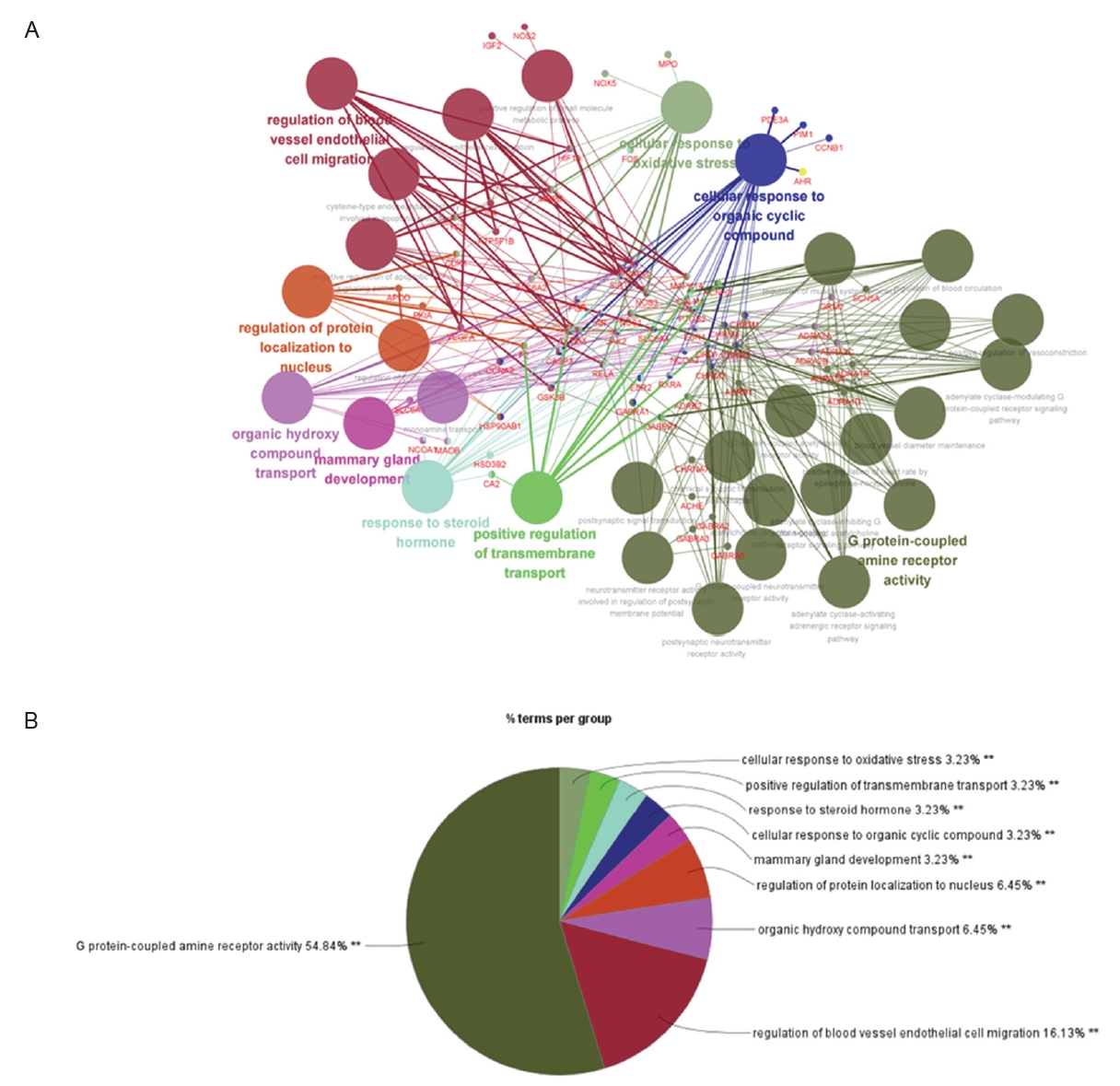
To test whether EB regulates lipogenesis, we assessed the cell viability and 3D holograms. As shown in Figure 2A, HepG2 cells were treated with diverse concentrations of EB (1, 10, 100, 1,000 and 10,000 ╬╝g/mL) for 24 h. Cell viability was not toxicity up to 1,000 ╬╝g/mL. Next, we confirmed that EB downregulates PA-induced lipid accumulation in HepG2 cells. As shown in the Figure 2B, palmitic acid (PA) increased the lipid accumulation to 0.16 ╬╝m3 ┬▒ 0.02 ╬╝m3, as compared to the untreated group. Whereas the EB significantly reduced the lipid accumulation to 0.12 ╬╝m3 ┬▒ 0.01 ╬╝m3 compared to the group treated with only PA.
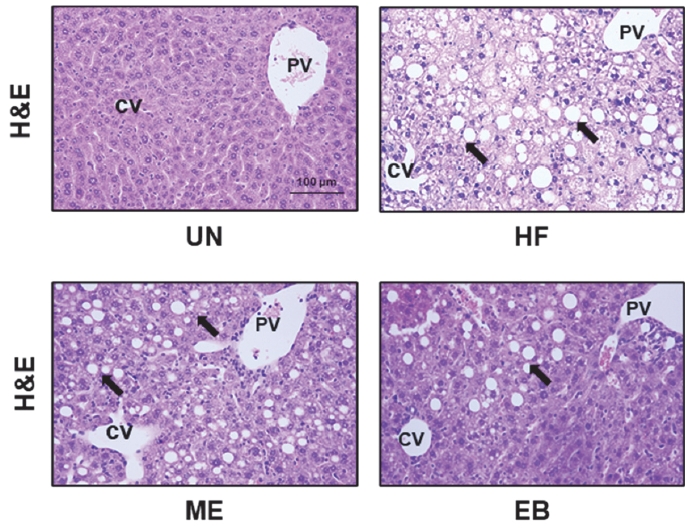
To investigate whether EB can control lipid accumulation in mice, mice were fed a high-fat diet for 24 weeks and were administered a combination of a high-fat diet and EB for an additional 8 weeks. The liver tissues were then stained with hematoxylin and eosin. As shown in Figure 3, the HF group had more mesh-like cells than the UN group. Furthermore, the EB and ME groups had fewer mesh-like cells than the HF group.
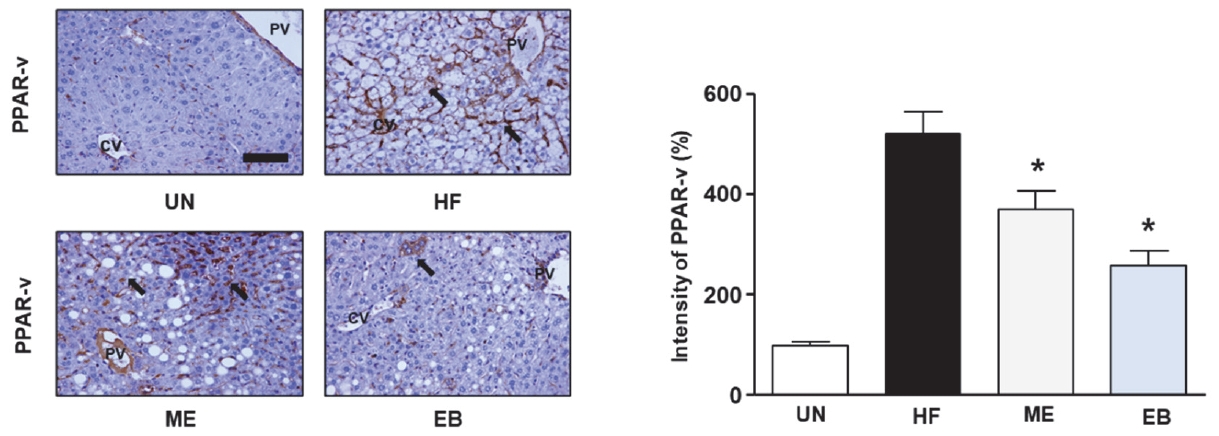
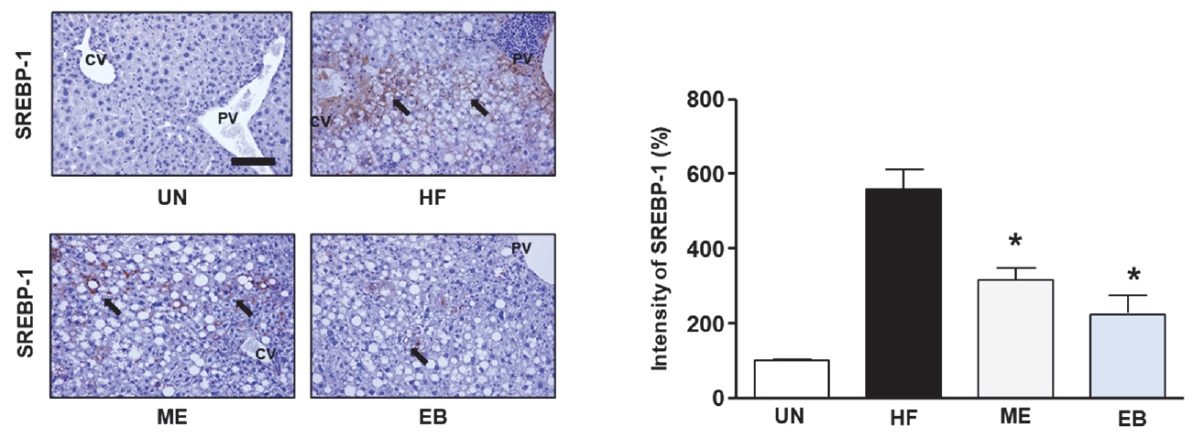
To explore EBŌĆÖs potential in regulating lipid accumulation in mice, we assessed the expression levels of SREBP-1 and PPAR-╬│ using immunohistochemistry and ImageJ. As shown in Figure 4, HF diet increased the expression of PPAR-╬│ up to 520 % ┬▒ 43.59 % compared with UN group. However, the ME and EB group indicated the expression of PPAR-╬│ to 370.0 % ┬▒ 36.06 % and 256.6 % ┬▒ 30.5 %, respectively. Moreover, the ME and EB group showed the expression of SREBP-1 to 316.67 % ┬▒ 30.55 % and 266.6 % ┬▒ 47.2 %, respectively (Figure 5).
In the present study, we investigated whether EB regulates lipogenesis both in vitro and in vivo. We also explored the possibility of using a systemic pharmacological approach to treat EB and performed a feasibility experiment to prove its effect in vivo. Lipid accumulation in PA-treated HepG2 cells was measured using 3D tomography. EB significantly regulates lipid accumulation. Furthermore, we explored the expression of SREBP-1 and PPAR-╬│ in liver tissue using immunohistochemistry, respectively. EB reduced the expression of SREBP-1 and PPAR-╬│ in the liver tissues of high-fat diet mice models. Both SREBP-1 and PPAR-╬│ are genes that promote lipogenesis. Consequently, we propose that EB is a potential candidate for NAFLD due to its ability to inhibit lipid accumulation by downregulating SREBP-1 and PPAR-╬│ expression.
Scorletti et al. reported that the continuous storage of lipid droplets induces NFALD 15 . As shown in Figure 2, our results showed that induced lipid droplets in HepG2 cells were treated with PA using Tomocube, whereas EB significantly reduced the PA-induced lipid droplets. Therefore, we sought to determine whether EB regulates lipid droplet production.
PPAR-╬│ and SREBP-1 are key factors that can regulate lipogenesis in vivo [19,20]. PPAR-╬│ in the liver of NAFLD patients was to be increased in the liver of NAFLD patients [21]. According to Kaneto et al., ME exhibits potential in the management of type 2 diabetes. ME activates AMPK in the liver, suppressing fatty acid synthesis and gluconeogenesis. Inhibition of lipid synthesis leads to the regulation of protein expression, including SREBP-1 and PPAR-gamma [22]. Pioglitazone, which is used to treat diabetes, is also prescribed for patients with NAFLD [23]. Moslehi et al. reported that the transcription factor SREBP-1 is involved in disorders such as obesity, atherosclerosis, and NAFLD [24]. As shown in Figures 4 and 5, EB significantly regulates the expression of SREBP-1 and PPAR-╬│, which are important factors in lipid metabolism. These results suggest that EB can reduce PPAR-╬│ and SREBP-1 expression.
Using the systems pharmacology analysis tool TCMSP, we identified 15 active substances in EBs. Among these 15 substances, formononetin, p-coumaric acid, protocatechualdehyde, isoliquiritigenin, baicalein, and cedar acid had the strongest antioxidant effects. Hence, we posit that EB enhances exercise performance. Moreover, in vivo ROS trigger AMPK activity and the activation of SREBP-1 and PPAR-╬│, culminating in lipid accumulation [25]. Illesca P et al. reported hydroxytyrosol, which has a strong antioxidant effect, consequently, be effective in NAFLD by regulating the signals of NK-kB, Nrf2, SREBP-1, and PPAR-gamma [26]. Our findings validate that EB, comprising diverse antioxidant compounds, modulates the expression of SREBP-1 and PPAR-╬│. Thus, EB may ameliorate NAFLD. Furthermore, the continuous production of ROS induces fatigue in vivo. Finanu et al. reported that oxidative stress is involved in muscle fatigue [27]. We also suggest that EB may have a synergistic effect on improving exercise performance.
In conclusion, our results confirmed that EB inhibits triglyceride synthesis in vitro and effectively regulates lipogenic factors, even in high-fat diet animal groups. In addition, the results of systems pharmacology analysis showed that EB contains various antioxidant substances and is a candidate supplement that can improve exercise capacity.
This supplement is essential for athletes across weight classes to enhance both athletic performance and weight control. Nevertheless, additional research is required to validate the synergistic effects of combining EB intake with exercise in models of NAFLD.
Acknowledgments
This result was supported by ŌĆ£Regional Inovation Strategy (RIS)ŌĆØ through the National Research Foundation of Korea (NRF) funded by the Ministry of education (MOE), 2021RIS-001(1345370811).
Figure┬Ā1.
Analysis of network pharmacology-based potential targets using gene ontology in Erigeron breviscapus (EB). (A) The large circles show the gene ontology using the Cytoscape. The red letters indicate the EB related-genes. (B) This graph represents the genetic ontology of EB.
Figure┬Ā2.
Effect of Erigeron breviscapus (EB) on the accumulation of lipid in HepG2 cells. (A) The cells seeded at 96 well plate and treated with diverse concentration of EB. The cell viability was measured using the WST-1 reagent. (B) The cells were treated with or without palmitic acid (PA) and EB. The lipid volume was measured using the lipid analysis software. Data in graph are presented as mean ┬▒ standard error (n = 3). *p < 0.05 vs untreated group. # p < 0.05 vs PA only treated group. UN; untreated group.

Figure┬Ā3.
Histological analysis of high fat diet-induced mice liver. The untreated group (UN) was provided with a standard diet for 32 weeks. Animal models were created by administering a high-fat diet for 24 weeks, and then the mice were divided the three groups following; HF group: high fat diet for 24 weeks after; ME group: high fat diet with positive control (17 mg of metformin /kg/day) for 8-week, EB group: high fat diet with natural products (26 mg of Erigeron breviscapus /kg/day) for 8 weeks. The tissues were stained with hematoxylin and eosin and observed using the microscopy. The block arrow indicates the hepatocyte with lipid drop. CV, Central vein; PV, Portal vein.
Figure┬Ā4.
Effect of EB on PPAR-╬│ expression in high fat diet mice. The untreated group (UN) was provided with a standard diet for 32 weeks. Animal models were created by administering a high-fat diet for 24 weeks, and then the mice were divided the three groups following; HF group: high fat diet for 24 weeks after; ME group: high fat diet with positive control (17 mg of metformin /kg/day) for 8-week, EB group: high fat diet with natural products (26 mg of Erigeron breviscapus /kg/day) for 8 weeks. The tissues were stained with anti-PPAR-╬│ and observed using the microscopy. The bar graph indicates that the expression of PPAR-╬│. Data in graph are presented as mean ┬▒ standard error. *p < 0.05 versus the HF-diet group. The block arrow indicates the PPAR-╬│ positive area. CV, Central vein; PV, Portal vein.
Figure┬Ā5.
Effect of EB on SREBP-1 expression in high fat diet mice. The untreated group (UN) was provided with a standard diet for 32 weeks. Animal models were created by administering a high-fat diet for 24 weeks, and then the mice were divided the three groups following; HF group: high fat diet for 24 weeks after; ME group: high fat diet with positive control (17 mg of metformin /kg/day) for 8-week, EB group: high fat diet with natural products (26 mg of Erigeron breviscapus /kg/day) for 8 weeks. The tissues were stained with anti-SREBP-1 and observed using the microscopy. The bar graph indicates that the expression of SREBP-1. Data in graph are presented as mean ┬▒ standard error. *p < 0.05 versus the HF-diet group. The block arrow indicates the SREBP-1 positive area. CV, Central vein; PV, Portal vein.
Table┬Ā1.
Analysis results of Erigeron breviscapus active ingredients from TCMSP database.
REFERENCES
1. Ghosh S, Paul M, Mondal KK, Bhattacharjee S, Bhattacharjee P. Sedentary lifestyle with increased risk of obesity in urban adult academic professionals: an epidemiological study in West Bengal, India. Sci Rep 2023;13:4895.




2. Feneberg A, Malfertheiner P. Epidemic trends of obesity with impact on metabolism and digestive diseases. Dig Dis 2012;30:143-7.


3. Paschos P, Paletas K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia 2009;13:9-19.


4. Kasper P, Martin A, Lang S, K├╝tting F, Goeser T, Demir M, Steffen HM. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol 2021;110:921-37.



5. Puengel T, Liu H, Guillot A, Heymann F, Tacke F, Peiseler M. Nuclear receptors linking metabolism, inflammation, and fibrosis in nonalcoholic fatty liver disease. Int J Mol Sci 2022;23:2668.



6. Wang YD, Wu LL, Qi XY, Wang YY, Liao ZZ, Liu JH, Xiao XH. New insight of obesity-associated NAFLD: dysregulated ŌĆ£crosstalkŌĆØ between multi-organ and the liver? Genes Dis 2022;10:799-812.



8. Berardo C, Di Pasqua LG, Cagna M, Richelmi P, Vairetti M, Ferrigno A. Nonalcoholic fatty liver disease and non-alcoholic steatohepatitis: current issues and future perspectives in preclinical and clinical research. Int J Mol Sci 2020;21:9646.



9. van der Windt DJ, Sud V, Zhang H, Tsung A, Huang H. The effects of physical exercise on fatty liver disease. Gene Expr 2018;18:89-101.



11. Fajas L, Schoonjans K, Gelman L, Kim JB, Najib J, Martin G, Fruchart JC, Briggs M, Spiegelman BM, Auwerx J. Regulation of peroxisome proliferator-activated receptor gamma expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: implications for adipocyte differentiation and metabolism. Mol Cell Biol 1999;19:5495-503.


12. Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. PPAR╬│ signaling and metabolism: the good, the bad and the future. Nat Med 2013;19:557-66.



13. Adegbola P, Aderibigbe I, Hammed W, Omotayo T. Antioxidant and anti-inflammatory medicinal plants have potential role in the treatment of cardiovascular disease: a review. Am J Cardiovasc Dis 2017;7:19-32.


14. Guo X, Li R, Cui J, Hu C, Yu H, Ren L, Cheng Y, Jiang J, Ding X, Wang L. Induction of RIPK3/MLKL-mediated necroptosis by Erigeron breviscapus injection exhibits potent antitumor effect. Front Pharmacol 2023;14:1219362.



15. Lee KP, Kim K, Yoon E, Baek S, Ahn SH. Pharmacological systemic analysis of gardenia fructus against non-alcoholic fatty liver disease and validation of animal models. Phys Act Nutr 2022;26:39-45.




16. Baek S, Kim J, Moon BS, Park SM, Jung DE, Kang SY, Lee SJ, Oh SJ, Kwon SH, Nam MH, Kim HO, Yoon HJ, Kim BS, Lee KP. Camphene attenuates skeletal muscle atrophy by regulating oxidative stress and lipid metabolism in rats. Nutrients 2020;12:3731.



17. Kim J, Lee KP, Beak S, Kang HR, Kim YK, Lim K. Effect of black chokeberry on skeletal muscle damage and neuronal cell death. J Exerc Nutrition Biochem 2019;23:26-31.



18. Scorletti E, Carr RM. A new perspective on NAFLD: focusing on lipid droplets. J Hepatol 2022;76:934-45.


19. Chen H, Tan H, Wan J, Zeng Y, Wang J, Wang H, Lu X. PPAR-╬│ signaling in nonalcoholic fatty liver disease: pathogenesis and therapeutic targets. Pharmacol Ther 2023;245:108391.


20. Aragno M, Tomasinelli CE, Vercellinatto I, Catalano MG, Collino M, Fantozzi R, Danni O, Boccuzzi G. SREBP-1c in nonalcoholic fatty liver disease induced by Western-type high-fat diet plus fructose in rats. Free Radic Biol Med 2009;47:1067-74.


21. Pettinelli P, Videla LA. Up-regulation of PPAR-gamma mRNA expression in the liver of obese patients: an additional reinforcing lipogenic mechanism to SREBP-1c induction. J Clin Endocrinol Metab 2011;96:1424-30.

22. Lin Y, Ren N, Li S, Chen M, Pu P. Novel anti-obesity effect of scutellarein and potential underlying mechanism of actions. Biomed Pharmacother 2019;117:109042.


23. Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem 1995;270:12953-6.

24. Moslehi A, Hamidi-Zad Z. Role of SREBPs in liver diseases: a mini-review. J Clin Transl Hepatol 2018;6:332-8.



25. Ma Y, Lee G, Heo SY, Roh YS. Oxidative stress is a key modulator in the development of nonalcoholic fatty liver disease. Antioxidants 2021;11:91.



26. Illesca P, Valenzuela R, Espinosa A, Echeverr├Ła F, Soto-Alarcon S, Ortiz M, Videla LA. Hydroxytyrosol supplementation ameliorates the metabolic disturbances in white adipose tissue from mice fed a high-fat diet through recovery of transcription factors Nrf2, SREBP-1c, PPAR-╬│ and NF-╬║B. Biomed Pharmacother 2019;109:2472-81.


- TOOLS






