 |
 |
- Search
| Phys Act Nutr > Volume 27(3); 2023 > Article |
|
Abstract
[Purpose]
This review aimed to investigate the effects of vitamin C and glutathione supplementation on exercise performance.
[Methods]
We conducted a literature search across the PubMed, Google Scholar, and Web of Science databases using the keywords vitamin C, glutathione, antioxidants, exercise, and oxidative stress.
[Results]
The effects of vitamin C supplementation on exercise performance and oxidative stress levels are inconsistent. Glutathione, with its diverse forms of supplementation and methods, presents mixed outcomes. Vitamin C and glutathione have deeply interconnected antioxidant functions and are mutually essential to each other. Research investigating the combined intake of these two substances, which are intricately linked biochemically, and their effects on exercise performance remain largely unexplored.
[Conclusion]
Studies on the effects of vitamin C and glutathione intake on exercise have been conducted using diverse approaches; however, the results have not been consistent. Although an additive effect is anticipated with the combined intake of vitamin C and glutathione, research on this topic is currently insufficient, and further studies are required.
Physical exercise has numerous health benefits, including improvements in cardiovascular fitness, muscular strength, and body composition. However, high-intensity training undertaken by athletes to enhance their performance leads to the generation of reactive oxygen species (ROS) that surpass the body’s antioxidant defense system, resulting in oxidative stress [1-3]. Oxidative stress can result in immune dysfunction and muscle damage, ultimately impairing athletic performance [4]. Increasing antioxidant levels in the bloodstream can help mitigate cellular oxidative stress [5]. Among antioxidants, vitamin C and glutathione are exceptionally potent and are biochemically intricately interconnected. Vitamin C is an essential, water-soluble vitamin with potent antioxidant properties. It plays a crucial role in neutralizing ROS and preventing oxidative damage to cells and tissues [6]. Vitamin C is involved in collagen synthesis, immune function, and iron absorption. Glutathione, a tripeptide composed of cysteine, glutamate, and glycine, is the most abundant antioxidant in cells. It acts as a potent scavenger of ROS and protects against oxidative stress-induced cellular damage. Additionally, glutathione plays a vital role in the detoxification of xenobiotics and the maintenance of the redox balance within cells [7]. Vitamin C and glutathione transform from their reduced forms to their oxidized forms, facilitating the elimination of free radicals. The oxidized forms are subsequently reduced back to their original state by reductive enzymes, allowing recycling within the antioxidant system. In this process, vitamin C and glutathione mutually rely on each other [8]. Herein we aimed to review the existing literature on the effects of vitamin C and glutathione supplementation on exercise performance, oxidative stress, and muscle damage.
Vitamin C, also known as ascorbic acid, is a water-soluble vitamin that plays a crucial role in the maintenance of overall health. Vitamin C has been extensively studied for its potential health benefits, including managing common cold symptoms [9,10], enhancement of immune function [11,12], and aiding in collagen synthesis [13,14]. Additionally, it may have positive effects on cardiovascular health [15-17] and improve iron absorption [18,19]. It is an essential nutrient as the human body cannot produce or store it in significant amounts, necessitating regular dietary intake. One of the primary functions of vitamin C is its potent antioxidant activity. As an antioxidant, it protects cells from the damage caused by free radicals. Free radicals are highly reactive and can cause oxidative stress, which has been linked to various chronic diseases, such as cardiovascular diseases, certain cancers, and age-related macular degeneration [20].
Ascorbate is the reduced active form of vitamin C. It acts as a powerful antioxidant by readily donating electrons to neutralize free radicals and ROS. By donating an electron, ascorbate stabilizes and neutralizes these harmful molecules, thereby protecting cells and tissues from oxidative damage. This process converts ascorbate into its oxidized form known as dehydroascorbate (Figure 1). Dehydroascorbate is the oxidized form of ascorbate. Dehydroascorbate can be transported across cell membranes, thereby allowing it to move between different cellular compartments. Once inside the cell, dehydroascorbate is enzymatically reduced to ascorbate, regenerating the active form of vitamin C. The interconversion between ascorbate and dehydroascorbate is an essential part of the antioxidant defense system. Ascorbate serves as a primary antioxidant, directly neutralizing free radicals, whereas dehydroascorbate acts as a mediator. The balance between ascorbate and dehydroascorbate is crucial for the overall antioxidant function of vitamin C. Vitamin C plays a vital role in recycling other antioxidants such as vitamin E. Vitamin E, a fat-soluble antioxidant, protects cell membranes from oxidative damage. After vitamin E neutralizes the free radicals, it is oxidized. Vitamin C can then interact with the oxidized form of vitamin E and restore its antioxidant function, allowing it to continue protecting cells [21]. Excessive consumption of vitamin C, that is, exceeding 1 g/day, may lead to adverse effects, such as gastrointestinal disturbances, metabolic acidosis, kidney stones, fatigue, and infertility. However, these effects are associated with the intake of vitamin C exceeding 1 g over several weeks [22,23]. However, supplementation with daily doses of 0.2 g or more but less than 1 g of vitamin C successfully reduces oxidative stress [24,25].
Vitamin C supplements are widely utilized by athletes because of their potential to enhance muscle recovery after intense exercise, which is attributed to their antioxidative effects [26,27]. Various studies have been conducted on vitamin C supplementation and exercise; however, the results have been inconsistent. In a study conducted by Thompson et al. on healthy males, a 2-week supplementation of vitamin C demonstrated beneficial effects on muscle soreness and function, as well as a significant reduction in plasma interleukin-6 (IL-6) levels following 90 minutes of prolonged intermittent shuttle-running test, compared to the placebo group [28]. This finding suggests the effectiveness of vitamin C supplementation in aiding post-exercise recovery. Bryer and Goldfarb observed that a 2-week vitamin C supplementation regimen in healthy males, followed by 4 days of eccentric exercise increased the oxidized glutathione (GSSG)/reduced glutathione (GSH) ratio, thereby preventing exercise-induced oxidative stress compared to the placebo group [29]. Evans et al. reported that 28-day vitamin C supplementation increased the peak muscular pushing force and reduced exercise-induced oxidative stress [30]. Other studies have reported the positive effects of vitamin C supplementation on the reduction of exercise-induced oxidative stress [31-33]; however, some studies have also shown that vitamin C supplementation has no impact or even negative effects on exercise performance and training adaptations. Gomez-Cabrera et al. reported a 9.3% reduction in maximal oxygen uptake with the consumption of 1,000 mg of vitamin C for 8 weeks [34]. Braakhuis et al. reported that 3 weeks of vitamin C supplementation during training decreases mean running speed in a 5 km running time in female runners [35]. In contrast, Roberts et al. reported that daily vitamin C supplementation (1000 mg/day) during 4 weeks of interval training did not interfere with training adaptation [36]. Several studies have investigated the effects of combining vitamin C with other antioxidants. Jourkesh et al. found that 3 weeks of 400 mg of vitamin E along with 1000 mg of vitamin C was effective in improving aerobic power [37]. Male athletes supplemented with an antioxidant complex, including vitamin C, exhibited higher training adherence [38,39]. However, several studies have reported that vitamin C and/or E supplementation does not improve exercise performance [40-43]. This discrepancy may be due to differences in participant status, exercise protocol, an-tioxidant dosage, form of intake, duration of intake, timing of intake, and methods for assessing oxidative stress [44]. In a review of vitamin C, vitamin E, and exercise adaptation, Nikolaidis et al. underscored the difficulty in determining the impact of vitamins C and E intake on chronic exercise adaptation and emphasized the need for future research involving appropriate exercise stimulus selection and reliable oxidative-reduction biomarker analyses at various post-exercise time points. Furthermore, they highlighted the need for studies evaluating antioxidant supplementation in welltrained individuals and employing effective and trustworthy testing protocols [45].
Glutathione, a tripeptide composed of glutamate, cysteine, and glycine, is the cornerstone of the antioxidant defense system. Its ability to counteract oxidative stress stems from its dual role as a direct scavenger of ROS and a critical regulator of cellular redox balance. Glutathione protects antioxidants by donating electrons to neutralize harmful free radicals, thus preventing oxidative damage to cellular components, such as proteins, lipids, and DNA [46]. Glutathione operates in an intricate cycle with enzymatic partners such as glutathione peroxidase and glutathione reductase. These enzymes facilitate the conversion of glutathione into its oxidized form (GSSG) and subsequently regenerate the reduced form (GSH) from GSSG. This cycle ensures a continuous supply of reduced glutathione, amplifying its capacity to combat oxidative stress and maintain cellular health [7]. Glutathione has a broad influence on cellular functions. It plays a pivotal role in modulating signaling pathways, gene expression, and immune responses, thereby influencing cellular adaptation to stress and maintaining overall homeostasis [47]. The recently reported roles of GSH in defending cells against oxidative stress and its involvement in protein modification and cellular signaling highlight the importance of the maintenance of intracellular GSH for cellular function and viability [48,49]. The decline in glutathione levels in the plasma and tissues has been linked to aging and disease. Glutathione depletion is particularly linked to neurodegenerative disorders, pulmonary diseases, immune disorders, cardiovascular diseases, and chronic geriatric conditions [50].
With the established association between glutathione levels and aging and disease, various studies have been conducted to explore methods for elevating glutathione levels. The effectiveness of oral glutathione in increasing intracellular glutathione levels has been much debated [51,52]. Some studies have indicated that acute or chronic oral glutathione does not significantly affect glutathione levels or oxidative stress. Witsch et al. found that oral supplementation with 3 g/day GSH did not alter blood GSH levels, while Allen et al. found no change in blood GSH levels or oxidative stress with 500 mg of glutathione supplementation twice daily for 4 weeks [53,54]. However, the evidence is conflicting. Richie et al. conducted a study involving 54 healthy adult males and reported that long-term oral glutathione (GSH) supplementation led to an increase in GSH levels in both blood and red blood cells and a reduction in oxidative stress [55]. Similarly, Park et al. found that, while oral glutathione supplementation did not induce changes in GSH levels in the plasma and cellular compartments, a significant increase was observed in the plasma protein-bound fraction [56]. Kovacs-Nolan et al. investigated the transportability of glutathione in intestinal epithelial cells in vitro. They showed that intestinal epithelial cells can transport glutathione. This challenges the previous notion and suggests that oral glutathione may not undergo significant degradation during digestion. Their findings contradict the theory that oral glutathione breaks down during digestion because of its inability to pass through these cells [57]. Owing to conflicting data regarding oral glutathione supplementation, attention has been directed toward the liposomal [58] or sublingual [59] forms of glutathione. The supplementation of NAC (N-acetylcysteine), which is essential for glutathione synthesis, has been suggested to increase glutathione levels. NAC has shown promise as a supplement for elevating glutathione levels and potentially alleviating certain issues associated with oxidative stress [60,61]. However, research on this topic is inconclusive, and some studies have reported minimal impact of NAC supplementation [62-65]. There is no officially established daily intake of glutathione; however, when used as an ingredient in pharmaceuticals or health functional foods, daily doses of approximately 50-500 mg are commonly used. Several clinical studies have indicated that a daily intake of 250-1000 mg can have significant beneficial effects [58,66]. Furthermore, recent clinical trials administering oral glutathione supplements (250 and 1000 mg/day) for 1, 3, and 6 months demonstrated doseand time-dependent increases in blood glutathione levels. In particular, long-term supplementation with 1000 mg/day for 6 months increased glutathione levels in the plasma, red blood cells, and oral cells, with no reported serious adverse effects [55]. Dilokthornsakul et al. reported minor side effects, such as a feeling of heaviness, reduced skin moisture, and flushing [67], but these effects were not severe. It is worth noting that some glutathione is synthesized in the body, and excessively consumed glutathione is excreted through the urine [68].
During exercise, heightened metabolic demands and increased oxygen consumption can lead to ROS generation in skeletal muscle cells. In the primary defense mechanism, glutathione serves as a key antioxidant by neutralizing ROS and quenching oxidative damage [7]. However, prolonged or intense physical activity can deplete intracellular GSH levels because GSH is oxidized to its disulfide form, glutathione disulfide (GSSG), during ROS detoxification [69,70]. The reduction in GSH availability may contribute to oxidative stress and muscle fatigue, potentially compromising exercise performance and recovery. To address the decrease in GSH levels due to exercise, researchers have investigated the efficacy of GSH supplements in enhancing exercise performance and mitigating oxidative stress. However, only a limited number of studies have been conducted on this topic. Aoi et al. demonstrated that healthy males who engaged in 60 minutes of cycling at 40% of their HRmax intensity after glutathione supplementation exhibited a less pronounced decrease in plasma glutathione levels and suppressed the increase in blood lactate concentration compared with the placebo group. This improved muscular aerobic metabolism during exercise, consequently reducing muscle fatigue [66]. Petrov et al. reported that 24 elite swimmers supplemented with 250 mg of glutathione daily during six weeks of training showed a significant decrease in swim time trials compared with the controls [71]. Owing to negative perceptions surrounding oral glutathione intake, many researchers have focused on NAC supplementation or intravenous injections as alternatives to oral glutathione administration. Supplementation with NAC(1200 mg/day) for 7 days improved muscle fatigue during the graded exercise treadmill test and increased VO2max [72]. Kelly et al. reported that NAC ingestion 45 min before submaximal discontinuous cycling exercise reduced exercise-induced respiratory muscle fatigue [73]. Medved et al. administered intravenous NAC in endurance-trained individuals before and during cycling and found an increase in submaximal performance and a 26% increase in the time to exhaustion. They also reported increased muscle cysteine levels and GSH availability in the individuals [74]. However, despite the positive effects of NAC, Rhodes and Braakhuis concluded in their meta-analysis that the side effects of NAC supplementation increased with increasing doses and that it could no longer be recommended [75]. In light of recent studies suggesting the effectiveness of oral glutathione supplementation, further research is warranted to elucidate the potential benefits of oral glutathione intake over NAC supplementation in enhancing intracellular glutathione availability.
The interaction between vitamin Cand glutathione has been well established, suggesting that they play a central role in cellular antioxidant defense systems. They are water-soluble and function in the cytoplasm and mitochondria [47,76-79]. The series of oxidation-reduction reactions that allow vitamin C and glutathione to interact each other makes them unique. The cyclic reactions are shown in Figure 3. The regeneration of the reduced compounds through this interconnected series of reactions amplifies the antioxidant capabilities of each compound. Without this redox coupling, the removal of oxidants would be constrained by the concentration of each compound. Mårtensson and Meister induced glutathione deficiency in adult mice by inhibitor administration. They observed a rapid and substantial increase in ascorbate levels in the liver of mice. Subsequently, the ascorbate levels decreased, whereas dehydroascorbate levels significantly increased. This suggests that glutathione deficiency hinders the reduction of dehydroascorbate, sustaining its elevated levels. Similar trends were observed in the kidneys and lungs, where ascorbate levels decreased, and dehydroascorbate levels increased. Therefore, during oxidative stress, glutathione depletion enhances ascorbate synthesis to protect against oxidative damage [80].
Beyond their biochemical interconnection, the profound association between vitamin C and glutathione is evident through research demonstrating changes in their levels in the blood or tissue upon vitamin C and/or glutathione supplementation. Vitamin C supplementation for 3 weeks in 48 individuals with ascorbate deficiency resulted in an 18% increase in lymphocyte glutathione levels compared with the placebo group [81]. Johnston et al. reported that vitamin C supplementation led to increased plasma glutathione concentration, regardless of the dosage (500 or 2000 mg/day). They concluded that vitamin C supplementation increased plasma GSH levels, thereby enhancing the protective antioxidant effect in the blood [82]. Ascorbate administration to newborn rats with glutathione deficiency has been shown to reduce toxicity and mortality rates and increase tissue and mitochondrial glutathione levels [83].
Despite the association between glutathione and vitamin C, studies investigating their combined intake are scarce. Sastre et al. administered glutathione, vitamin C, or NAC to rats orally for 7 days. In the control group, a significant increase in GSSG was observed during exhaustive exercise; however, the increase in GSSG in the supplementation groups was attenuated. As part of the same study, national-level elite athletes were administered 1 g of glutathione and 2 g of vitamin C orally for 7 days, and changes in serum GSH, GSSG, and the GSSG/GSH ratio were assessed before and after exhaustive exercise. Positive improvements in the GSSG/GSH ratio were observed immediately after exhaustive exercise following the intervention, compared to pre-intervention levels. This is valuable as it is the first report of the effects of combined vitamin C and glutathione supplementation on serum glutathione levels after exercise. However, owing to the small sample size of only 5 participants, the results were not statistically analyzed; moreover, there was no control group. Additionally, this study did not examine parameters other than serum glutathione, making it challenging to precisely ascertain the effects of combined vitamin C and glutathione supplementation [84]. We recently studied the effects of combined glutathione and vitamin C supplementation using an improved study design. We found that compared to vitamin C or glutathione single supplementation, acute vitamin C and glutathione complex supplementation in 12 middle-aged triathletes resulted in decreased carbon dioxide output, decreased heart rate, and positive improvements in skeletal muscle oxygenation during 90 min of cycling exercise at 70% VO2max, as well as positive improvements in blood and biological antioxidant potential (BAP) and diacron reactive oxygen metabolite(dROM) [85]. Except for the two papers mentioned above, no specific studies on the exercise performance effects of combined vitamin C and glutathione supplementation were found in the database search. However, one study investigated the effect of combined supplementation of vitamin C with NAC and zinc on exercise performance. Alduraywish conducted a study involving 18 male novice volleyball players who were administered a combination of 750 mg NAC (a precursor of glutathione), 5 mg zinc, and 100 mg vitamin C for 7 days. The effects of this supplementation on time-to-exhaustion (TTE), cardiorespiratory fitness (CRF) index, and metabolic indicators were investigated. The results indicated significant improvements in TTE and CRF following one-week supplementation with the combination of substances [83]. Although limited, previous studies have suggested that the combined supplementation of vitamin C and glutathione may exhibit additive effects compared to individual supplementation. Additional research and validation studies are necessary to determine precise dosage recommendations and evaluate the extended impacts across a wider spectrum of athletes and athletic scenarios.
Various studies have investigated the effects of antioxidant supplements, such as vitamin C or glutathione, on exercise performance. However, the diversity in study subjects, supplementation methods, and duration of intake has led to inconsistent findings. Vitamin C and glutathione are potent antioxidants that are intricately linked biochemically; it has been hypothesized that their combined supplementation could provide a additive effect in combating exercise-induced oxidative stress. However, research on combined supplementation remains limited, necessitating extensive studies for a more comprehensive understanding.
Figure 3.
Cyclical redox reactions that link glutathione (GSH) and ascorbic acid (AA). GSSG: glutathione disulfide, DHA: dehydroascorbate.
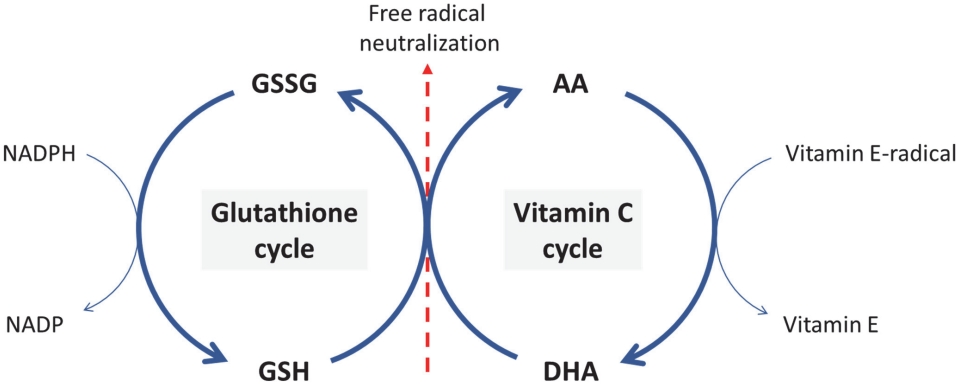
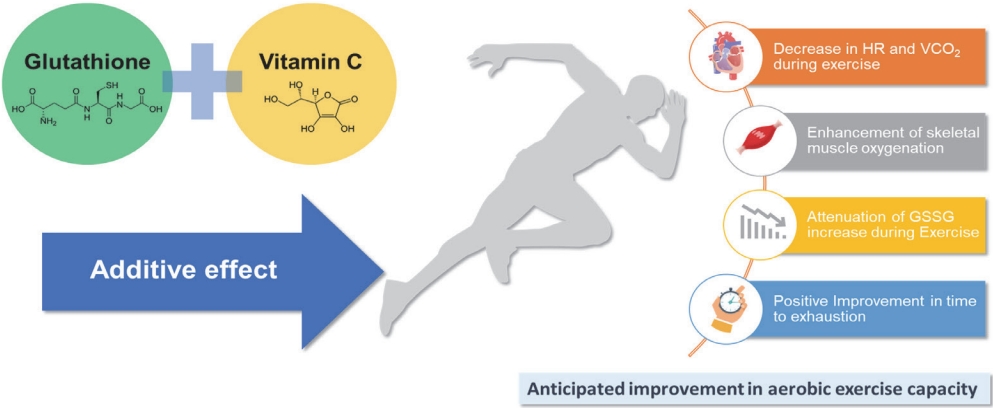
Figure 4.
Effect of combined glutathione and vitamin C supplementation. The combined supplementation of glutathione and vitamin C is expected to have a additive effect. According to previous studies, combined supplementation has been shown to reduce heart rate (HR) and carbon dioxide output (VCO2) during exercise of the same intensity, improve skeletal muscle oxygenation, attenuate the increase in glutathione disulfide (GSSG: oxidized form of glutathione) during exercise, and even extend exhaustion time. These findings suggest that the supplementation of this combination may have a positive impact on improving aerobic exercise capacity.
REFERENCES
1. Silva AN, Lima LCF. The association between physical exercise and reactive oxygen species (ROS) production. JSHS 2013;2:87-93.
2. Goldfarb AH, Bloomer RJ, McKenzie MJ. Combined antioxidant treatment effects on blood oxidative stress after eccentric exercise. Med Sci Sports Exerc 2005;37:234-9.


3. Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology 2003;189:41-54.


4. Watson TA, MacDonald-Wicks LK, Garg ML. Oxidative stress and antioxidants in athletes undertaking regular exercise training. Int J Sport Nutr Exerc Metab 2005;15:131-46.


5. Clarkson PM, Thompson HS. Antioxidants: what role do they play in physical activity and health? Am J Clin Nutr 2000;72:637S-46S.


7. Pizzorno J. Glutathione! Integr Med 2014;13:8-12.
8. Linster CL, Schaftingen EV. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J 2007;274:1-22.
9. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev 2013;2013:CD000980.


10. Straten MV, Josling P. Preventing the common cold with a vitamin C supplement: a double-blind, placebo-controlled survey. Adv Ther 2002;19:151-9.



12. Ang A, Pullar JM, Currie MJ, Vissers MCM. Vitamin C and immune cell function in inflammation and cancer. Biochem Soc Trans 2018;46:1147-59.




13. Shaw G, Lee-Barthel A, Ross ML, Wang B, Baar K. Vitamin C- enriched gelatin supplementation before intermittent activity augments collagen synthesis. Am J Clin Nutr 2017;105:136-43.



14. DePhillipo NN, Aman ZS, Kennedy MI, Begley JP, Moatshe G, LaPrade RF. Efficacy of vitamin C supplementation on collagen synthesis and oxidative stress after musculoskeletal injuries: a systematic review. Orthop J Sports Med 2018;6:2325967118804544.




15. Moser MA, Chun OK. Vitamin C and heart health: a review based on findings from epidemiologic studies. Int J Mol Sci 2016;17:1328.



16. Martín-Calvo N, Martínez-González MA. Vitamin C intake is inversely associated with cardiovascular mortality in a cohort of Spanish graduates: the SUN project. Nutrients 2017;9:954.



17. Morelli MB, Gambardella J, Castellanos V, Trimarco V, Santulli G. Vitamin C and cardiovascular disease: an update. Antioxidants 2020;9:1227.



18. Hallberg L, Brune M, Rossander L. The role of vitamin C in iron absorption. Int J Vitam Nutr Res Suppl 1989;30:103-8.

20. Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK, Levine M. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr 2003;22:18-35.


22. Braakhuis AJ. Effect of vitamin C supplements on physical performance. Curr Sports Med Rep 2012;11:180-4.


23. Griffiths HR, Lunec J. Ascorbic acid in the 21st century - more than a simple antioxidant. Environ Toxicol Pharmacol 2001;10:173-82.


24. Dekkers JC, van Doornen LJ, Kemper HC. The role of antioxidant vitamins and enzymes in the prevention of exercise-induced muscle damage. Sports Med 1996;21:213-38.


25. McGuire S. U.S. Department of Agriculture and U.S. Department of Health and Human Services, Dietary Guidelines for Americans, 2010. 7th Edition. Washington, DC: U.S. Government Printing Office 2011;2:293-4.
26. Braun H, Koehler K, Geyer H, Kleinert J, Mester J, Schänzer W. Dietary supplement use among elite young german athletes. Int J Sport Nutr Exerc Metab 2009;19:97-109.


28. Thompson D, Williams C, McGregor SJ, Nicholas CW, McArdle F, Jackson MJ, Powell JR. Prolonged vitamin C supplementation and recovery from demanding exercise. Int J Sport Nutr Exerc Metab 2001;11:466-81.


29. Bryer SC, Goldfarb AH. Effect of high dose vitamin C supplementation on muscle soreness, damage, function, and oxidative stress to eccentric exercise. Int J Sport Nutr Exerc Metab 2006;16:270-80.


30. Evans LW, Zhang F, Omaye ST. Vitamin C supplementation reduces exercise-induced oxidative stress and increases peak muscular force. Food Nutr Sci 2017;8:812-22.


31. Alessio HM, Goldfarb AH, Cao G. Exercise-induced oxidative stress before and after vitamin C supplementation. Int J Sport Nutr 1997;7:1-9.


32. Ashton T, Young IS, Peters JR, Jones E, Jackson SK, Davies B, Rowlands CC. Electron spin resonance spectroscopy, exercise, and oxidative stress: an ascorbic acid intervention study. J Appl Physiol 1999;87:2032-6.


33. Vasankari T, Kujala U, Sarna S, Ahotupa M. Effects of ascorbic acid and carbohydrate ingestion on exercise induced oxidative stress. J Sports Med Phys Fitness 1998;38:281-5.

34. Gomez-Cabrera MC, Domenech E, Viña J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Bio Med 2008;44:126-31.

35. Braakhuis AJ, Hopkins WG, Lowe TE. Effects of dietary antioxidants on training and performance in female runners. Eur J Sport Sci 2014;14:160-8.


36. Roberts LA, Beattie K, Close GL, Morton JP. Vitamin C consumption does not impair training-induced improvements in exercise performance. Int J Sports Physiol Perform 2011;6:58-69.


37. Jourkesh M, Ostojic SM, Azarbayjani MA. The effects of vitamin E and vitamin C supplementation on bioenergetics index. Res Sports Med 2007;15:249-56.


38. Aguiló A, Tauler P, Sureda A, Cases N, Tur J, Pons A. Antioxidant diet supplementation enhances aerobic performance in amateur sportsmen. J Sport Sci 2007;25:1203-10.

39. Gauche E, Lepers R, Rabita G, Leveque JM, Bishop D, Brisswalter J, Hausswirth C. Vitamin and mineral supplementation and neuromuscular recovery after a running race. Med Sci Sports Exerc 2006;38:2110-7.


40. de Oliveira DCX, Rosa FT, Simões-Ambrósio L, Jordao AA, Deminice R. Antioxidant vitamin supplementation prevents oxidative stress but does not enhance performance in young football athletes. Nutrition 2019;63:29-35.

41. Bloomer RJ, Falvo MJ, Schilling BK, Smith WA. Prior exercise and antioxidant supplementation: effect on oxidative stress and muscle injury. J Int Soc Sports Nutr 2007;4:9.




42. Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA 2009;106:8665-70.



43. Yfanti C, Nielsen AR, Åkerström T, Nielsen S, Rose AJ, Richter EA, Lykkesfeldt J, Fischer CP, Pedersen BK. Effect of antioxidant supplementation on insulin sensitivity in response to endurance exercise training. Am J Physiol Endocrinol Metab 2011;300:E761-70.


44. Peternelj TT, Coombes JS. Antioxidant supplementation during exercise training: beneficial or detrimental? Sports Med 2011;41:1043-69.

45. Nikolaidis MG, Kerksick CM, Lamprecht M, McAnulty SR. Does vitamin C and E supplementation impair the favorable adaptations of regular exercise? Oxid Med Cell Longev 2012;2012:707941.




46. Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med 2009;30:1-12.



48. Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A. S-glutathionylation in protein redox regulation. Free Radic Biol Med 2007;43:883-98.


50. Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem 2009;390:191-214.



52. Meister A. Glutathione deficiency produced by inhibition of its synthesis, and its reversal; applications in research and therapy. Pharmacol Ther 1991;51:155-94.


53. Witschi A, Reddy S, Stofer B, Lauterburg BH. The systemic availability of oral glutathione. Eur J Clin Pharmacol 1992;43:667-9.



54. Allen J, Bradley RD. Effects of oral glutathione supplementation on systemic oxidative stress biomarkers in human volunteers. J Altern Complement Med 2011;17:827-33.



55. Richie JP, Nichenametla S, Neidig W, Calcagnotto A, Haley JS, Schell TD, Muscat JE. Randomized controlled trial of oral glutathione supplementation on body stores of glutathione. Eur J Nutr 2015;54:251-63.



56. Park EY, Shimura N, Konishi T, Sauchi Y, Wada S, Aoi W, Nakamura Y, Sato K. Increase in the protein-bound form of glutathione in human blood after the oral administration of glutathione. J Agric Food Chem 2014;62:6183-9.


57. Kovacs-Nolan J, Rupa P, Matsui T, Tanaka M, Konishi T, Sauchi Y, Sato K, Ono S, Mine Y. In vitro and ex vivo uptake of glutathione (GSH) across the intestinal epithelium and fate of oral GSH after in vivo supplementation. J Agric Food Chem 2014;62:9499-506.


58. Sinha R, Sinha I, Calcagnotto A, Trushin N, Haley JS, Schell TD, Richie JP. Oral supplementation with liposomal glutathione elevates body stores of glutathione and markers of immune function. Eur J Clin Nutr 2018;72:105-11.



59. Campolo J, Bernardi S, Cozzi L, Rocchiccioli S, Dellanoce C, Cecchettini A, Tonini A, Parolini M, Chiara BD, Micheloni G, Pelosi G, Passino C, Giannattasio C, Parodi O. Medium-term effect of sublingual l-glutathione supplementation on flow-mediated dilation in subjects with cardiovascular risk factors. Nutrition 2017;38:41-7.


60. Jannatifar R, Parivar K, Roodbari NH, Nasr-Esfahani MH. Effects of N-acetyl-cysteine supplementation on sperm quality, chromatin integrity and level of oxidative stress in infertile men. Reprod Biol Endocrinol 2019;17:24.




61. Zhang J, Ye Z wei, Singh S, Townsend DM, Tew KD. An evolving understanding of the S-glutathionylation cycle in pathways of redox regulation. Free Radic Biol Med 2018;120:204-16.



62. Rushworth GF, Megson IL. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol Ther 2014;141:150-9.


63. Ellegaard PK, Licht RW, Nielsen RE, Dean OM, Berk M, Poulsen HE, Mohebbi M, Nielsen CT. The efficacy of adjunctive N-acetylcysteine in acute bipolar depression: a randomized placebo-controlled study. J Affect Disord 2019;245:1043-51.


64. Berk M, Turner A, Malhi GS, Ng CH, Cotton SM, Dodd S, Samuni Y, Tanious M, McAulay C, Dowling N, Sarris J, Owen L, Waterdrinker A, Smith D, Dean OM. A randomised controlled trial of a mitochondrial therapeutic target for bipolar depression: mitochondrial agents, N-acetylcysteine, and placebo. BMC Med 2019;17:18.




65. Panizzutti B, Bortolasci C, Hasebe K, Kidnapillai S, Gray L, Walder K, Berk M, Mohebbi M, Dodd S, Gama C, Magalhães PV, Cotton SM, Kapczinski F, Bush AI, Malhi GS, Dean OM. Mediator effects of parameters of inflammation and neurogenesis from a N-acetyl cysteine clinical-trial for bipolar depression. Acta Neuropsychiatr 2018;30:334-41.


66. Aoi W, Ogaya Y, Takami M, Konishi T, Sauchi Y, Park EY, Wada S, Sato K, Higashi A. Glutathione supplementation suppresses muscle fatigue induced by prolonged exercise via improved aerobic metabolism. J Int Soc Sports Nutr 2015;12:7.




67. Dilokthornsakul W, Dhippayom T, Dilokthornsakul P. The clinical effect of glutathione on skin color and other related skin conditions: a systematic review. J Cosmet Dermatol 2019;18:728-37.



68. Aebi S, Assereto R, Lauterburg BH. High-dose intravenous glutathione in man. Pharmacokinetics and effects on cyst(e)ine in plasma and urine. Eur J Clin Invest 1991;21:103-10.


69. Lew H, Pyke S, Quintanilha A. Changes in the glutathione status of plasma, liver and muscle following exhaustive exercise in rats. FEBS Lett 1985;185:262-6.



70. Gohil K, Viguie C, Stanley WC, Brooks GA, Packer L. Blood glutathione oxidation during human exercise. J Appl Physiol 1988;64:115-9.


71. Petrov L, Alexandrova A, Kachaunov M, Penov R, Sheytanova T, Kolimechkov S. Effect of glutathione supplementation on swimmers’ performance. Pedagogy Phys Cult Sports 2021;25:215-24.


72. Leelarungrayub D, Khansuwan R, Pothongsunun P, Klaphajone J. N-acetylcysteine supplementation controls total antioxidant capacity, creatine kinase, lactate, and tumor necrotic factor-alpha against oxidative stress induced by graded exercise in sedentary men. Oxid Med Cell Longev 2011;2011:329643.




73. Kelly MK, Wicker RJ, Barstow TJ, Harms CA. Effects of N-acetylcysteine on respiratory muscle fatigue during heavy exercise. Respir Physiol Neurobiol 2009;165:67-72.


74. Medved I, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X, McKenna MJ. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J Appl Physiol 2004;97:1477-85.


75. Rhodes K, Braakhuis A. Performance and side effects of supplementation with N-acetylcysteine: a systematic review and meta-analysis. Sports Med 2017;47:1619-36.



76. Meister A. Glutathione-ascorbic acid antioxidant system in animals. J Biol Chem 1994;269:9397-400.


77. Meister A. On the antioxidant effects of ascorbic acid and glutathione. Biochem Pharmacol 1992;44:1905-15.


78. Meister A. The antioxidant effects of glutathione and ascorbic acid. Oxidative Stress, Cell Activation and Viral Infection 1994;101-11.

79. Sies H, Stalhl W, Sundquist AR. Antioxidant functions of vitamins. Vitamins E and C, beta-carotene, and other carotenoids. Ann N York Acad Sci 1992;669:7-20.
80. Mårtensson J, Meister A. Glutathione deficiency increases hepatic ascorbic acid synthesis in adult mice. Proc Natl Acad Sci USA 1992;89:11566-8.



81. Lenton KJ, Sané AT, Therriault H, Cantin AM, Payette H, Wagner JR. Vitamin C augments lymphocyte glutathione in subjects with ascorbate deficiency. Am J Clin Nutr 2003;77:189-95.


82. Johnston CS, Meyer CG, Srilakshmi JC. Vitamin C elevates red blood cell glutathione in healthy adults. Am J Clin Nutr 1993;58:103-5.


83. Alduraywish AA. Cardiorespiratory and metabolic fitness indicators in novice volleyball trainees: effect of 1-week antioxidant supplementation with N-acetyl-cysteine/zinc/vitamin C. J Int Med Res 2021;49:03000605211067125.




84. Sastre J, Asensi M, Gasco E, Pallardo FV, Ferrero JA, Furukawa T, Vina J. Exhaustive physical exercise causes oxidation of glutathione status in blood: prevention by antioxidant administration. Am J Physiol 1992;263:R992-5.


85. Lee E, Park HY, Kim SW, Sun Y, Choi JH, Seo J, Jung YP, Kim AJ, Kim J, Lim K. Enhancing supplemental effects of acute natural antioxidant derived from yeast fermentation and vitamin C on sports performance in triathlon athletes: a randomized, double-blinded, placebo-controlled, crossover trial. Nutrients 2023;15:3324.



-
METRICS

-
- 0 Crossref
- Scopus
- 1,368 View
- 55 Download
- Related articles in Phys Act Nutr
-
Invite Review : Creatine supplementation in exercise, sport, and medicine2011 June;15(2)







