 |
 |
- Search
| Phys Act Nutr > Volume 27(2); 2023 > Article |
|
Abstract
[Purpose]
In the current study, we investigated the effects of camellia oil and camellia oil infused with herbs (Camellia oleifera Abel) on obesity in obese mice fed a high-fat diet (HFD).
[Methods]
The antioxidant activity of camellia oil in scavenging free radicals was investigated. Additionally, body and organ weight changes, serum and liver marker parameters, antioxidant enzyme activities, liver and epididymal fat histology, protein and gene expression associated with lipogenesis and hyperglycemia effect on adenosine monophosphate-activated protein kinase (AMPK) phosphorylation, were examined in HFD-induced obese mice.
[Results]
The hepatic steatosis and epididymal fat were significantly reduced by the oral administration of camellia oil and herb-infused camellia oil. Moreover, hepatic and serum marker parameters such as total cholesterol, insulin, triglycerides, tumor necrosis factor-α, adiponectin, thiobarbituric acid reactive substances, aspartate aminotransferase, and alanine transaminase were beneficially impacted. Additionally, the activity of antioxidant enzymes also increased. Camellia oil and herb-infused camellia oil treatments reduced the expression of genes linked to hyperglycemia and lipogenesis via activation of AMPK phosphorylation.
[Conclusion]
For many people, exercise poses an obstacle in the daily routine due to lack of ease, difficulty in maintaining consistency, and hard work. Camellia oil combined with herbs has anti-obesity and antihyperglycemic effects. These findings indicate that treatment with herb-infused camellia oil is most beneficial for elderly individuals who do not prefer frequent exercise.
Obesity is the excessive or abnormal accumulation of body fat due to a variety of factors, including lack of exercise, excessive food intake, and heredity [1]. Obesity is the sixth most common cause of mortality [2]. Moreover, it is well established that consuming excessive fat can result in an increase in body’s adipose tissues, which leads to obesity and an increase in the risk of several diseases, including diabetes, kidney disease, fatty liver disease, atherosclerosis, hypertension, and coronary heart disease [3-5]. A safe and effective preventive method is urgently needed due to the number of affected individuals [6].
Obesity causes excessive fat accumulation in the liver because adipose tissue has a limited capacity to store fat [7] which leads to production of reactive oxygen species (ROS) [8]. ROS are believed to cause liver macrophages to switch from an anti-inflammatory to a pro-inflammatory activation state, resulting in insulin resistance [9]. Preclinical research has used C57BL/6J mice as a polygenic model for diet-induced obesity because the mechanism of obesity in these mice is comparable to that in humans [10,11]. To better understand the pathogenic mechanisms of obesity-related disorders, high-fat diets (HFD), which comprise 45-60% dietary fat, are frequently utilized in animal disease models, such as those for diabetes, liver disease, and cardiovascular diseases [12-14]. Few anti-obesity medications are used in clinical settings and have substantial side effects [15,16]. Diet with regular exercise are important factors in the prevention of obesity. Herbal products and particular foods have significant potential to actively promote weight control because of their capacity to prevent fat accumulation and reduce body weight. This is because they increase thermogenesis, fat oxidation, and energy expenditure [17]. Therefore, understanding the regulatory mechanism of obesity (abiogenesis) is crucial for its prevention and the conceptualization of anti-obesity diets.
Camellia oil is a type of edible oil with strong medicinal and nutritional qualities used in China that is made from Camellia oleifera Abel seeds [18,19]. It contains high levels of carotene, vitamin E, polyphenols, and unsaturated fatty acids such as linoleic, palmitic, and oleic acids, all of which are crucial phytochemicals for fat metabolism [20]. Previous pharmacological investigations have claimed that camellia oil has anti-obesity, anti-inflammatory, and anti-weight-gain properties [20-23]. Importantly, several studies revealed that camellia seed oil had positive effects on liver protection and hypolipidemia in HFD-fed mice [24-26]. Additionally, the Food and Agriculture Organization suggested that camellia oil is a beneficial plant oil [27]. Rosemary (Rosmarinus officinalis L.) has been used to treat various chronic illnesses since ancient times. Current studies have demonstrated the potential of rosemary in the treatment of diabetes and obesity [28]. Rosemary contains high concentrations of phenolic diterpenes, triterpenes, phenolic acids, and flavonoids, which have antioxidant, anti-hyperlipidemic, and anti-hyperglycemic properties. Numerous studies have shown that rosemary consumption offers several health advantages, such as a reduced risk of obesity, diabetes, and other metabolic syndromes [29-32]. Similarly, Syzygium aromaticum (L.), often known as cloves, contains phytochemicals such as alkaloids, tannins, flavonoids, and phenols, which are responsible for some of its biochemical characteristics. Animals treated with S. aromaticum have been shown to benefit from its anti-diabetic, anti-inflammatory, antibacterial, aphrodisiac, and antioxidant capabilities [33-35]. A recent study investigated silicone-infused camellia seed oil as an anti-icing/frosting material for food-freezing applications [36].
According to the literature survey, camellia oil, rosemary and cloves have unique antioxidant capabilities with anti-inflammatory and anti-obesity effects. However, to compare the performance of a product infused with herbs (rosemary and cloves) to that of a product containing 100% camellia oil, scientific verification of the efficacy of the product is required as well as in vitro and in vivo testing for antioxidant activity and anti-obesity properties. However, the effects of camellia oil alone and herb-infused (rosemary and cloves) camellia oil on liver and fat inflammation in HFD-induced obese mice have not yet been studied. However, the current study investigated that the effects of camellia oil (Cam) and camellia oil infused with rosemary and cloves (Cam+Herb) on oxidative stress, obesity, and morphological changes in the liver and adipose tissues of C57BL/6J mice fed with a saturated fat-rich diet.
Camellia oil was purchased from an agricultural company, GOMARI Co., Ltd. (Yeosu-si, Jeollanam-do, Korea), and was made from camellia seeds and to prepare herb-infused camellia oil, the oil was infused with 5% rosemary and clove at a 1:1 ratio. For subsequent experimentation, camellia oil and herb-infused oil were kept in an airtight container at 4 ˚C for a maximum of one year.
Using 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals, the in vitro scavenging ability of camellia oil and herb-infused camellia oil was evaluated. We have modified the DPPH radical scavenging technique and described the DPPH radical scavenging activity analysis method [37]. Three replicate experiments were performed using 96-well plates. We prepared several quantities of camellia oil and herb-infused camellia oil (0.625, 1.25, 2.5, 5, and 10 mg/mL) along with a methanolic 0.208 mM DPPH solution. Next, 50 μL of each sample was combined with 150 μL of the DPPH radical solution. The reaction mixture containing 96-well plate was incubated at room temperature in complete darkness for 20 min before the absorbance was measured at 540 nm. The percentage (%) of DPPH radicals inhibited was determined by using the following formula: radical scavenging activity (RSA) = 100 × (Acontrol − Asample)/Acontrol, where Acontrol and Asample are absorbance values.
Male C57BL/6J mice aged five weeks (15-21 g body weight [BW]) were obtained from Orient Bio (Seongnam, Korea). The animals were housed in accordance with the Chonnam National University Guidelines for the Care and Use of Laboratory Animals. Before the study, they were acclimated for a week in a room with 55.5% humidity control and 12 h light/dark cycle.
This research is aimed to determine the effects of camellia oil and herb-infused camellia oil treatment. A dose of 5 mL/kg BW/day was administered for a period of seven weeks, following previously published studies18-26. In this study, after one week of acclimatization, the C57BL/6J mice were randomly classified into four groups: (1) HFD-induced obese group (CON); (2) standard diet-fed normal group (NOR); (3) HFD-induced obese group treated with camellia oil (Cam); and (4) HFD-induced obese group treated with camellia oil infused with herbs (Cam+Herb). Biochemical analyses of the CON and NOR group were compared. Moreover, the beneficial effects of camellia oil (Cam group) and camellia oil infused with herbs (Cam+Herb group) were analyzed and compared with the corresponding parameters in the CON group. Further, normal (NOR) mice were provided with feed that contained the following components per kg of food: t-butyl hydroquinone (0.01 g), vitamin mix (10 g), choline bitartrate (2.5 g), and mineral mix (35 g). The CON, Cam (orally treated Camellia oil with 5 mL/kg BW/day; Daily intake ratio: 0.52), and Cam+Herb (orally treated with Camellia oil infused with herbs at 5 mL/kg BW/day; daily intake ratio: 0.53) groups were received HFD comprising the following components per kg of diet: cysteine (176.8 g), sucrose (176.8 g), casein lactic acid (200 g), malt dextrin (100 g), corn starch (72.8 g), soybean oil (25 g), cellulose (50 g), animal fat (177.5 g), minerals (50 g), choline bitartrate (2 g), vitamin mix (1 g), and dye (0.05 g). During the experimental period, the daily dietary intake and weight changes were recorded between 08:00-09:00 hours. The food efficiency ratio (FER) was estimated by calculating the body weight gain (g/day) / the daily food intake (g/day) ×100, throughout the experiment.
After seven weeks, isoflurane (Hana Pharm Co., Ltd., Seongnamm, Korea) was used to anesthetize the mice through inhalation. Serum samples were obtained by collecting blood samples in heparin tubes and centrifuging them at 2,000 × g for 10 min at 4 ˚C to determine biochemical characteristics. The organs were collected, washed with physiological saline, dried, and weighed. They were rapidly frozen using liquid nitrogen and kept for examination at -80 ˚C. The weights of fat were obtained from various locations. Hepatic and epididymal fat tissues were preserved in 10% formalin for histological analysis. The liver tissues were homogenized at a ratio of 1:9 in 450 mM phosphate buffer (pH 7) and centrifuged at 25,000 rpm at 4 ˚C for 20 min. Then the supernatant was collected to analyze lipid and antioxidant activities. The experimental methods utilized in this study were approved by the Chonnam National University’s animal ethics committee (CNU IACUC-YS-2020-9).
An ELISA kit (Elabscience Inc., USA) was used to quantify insulin (INS), thiobarbituric acid reactive substances (TBARS), tumor necrosis factor-α (TNF-α), and adiponectin [38]. Enzymatic analysis kits (Asan Pharmaceuticals, Hwasung, Korea) were used to evaluate the levels of TC, high-density lipoproteins (HDL) cholesterol, low-density lipoproteins (LDL) cholesterol, and triglycerides (TG), as well as the activities of alanine transaminase (ALT) and aspartate transaminase (AST) [39]. Total protein (Biuret test kit, Elabscience Inc., USA), blood urine nitrogen (BUN) (urease GLDH test kit, Elabscience Inc., USA), and albumin (BCG kit, Elabscience Inc., USA) were measured [40].
The manufacturer’s instructions were followed to determine glutathione (GSH), glutathione reductase (GR), glutathione peroxidase (GPX), superoxide dismutase (SOD) and glutathione S-transferases (GST) activities in the liver tissue using a colorimetric test kit (Biovision Inc, San Francisco, CA, USA) [41].
Tissues were initially fixed with formalin (10% [v/v]) in phosphate buffer before being embedded in paraffin wax for histological investigation. Subsequently, H&E stain was applied to each of the 4-6 μm thick portions. A camera and optical microscope (Olympus DP70, Olympus Optical Co., Tokyo, Japan) were used to observe histological alterations [42].
With a few minor modifications, the procedures described in [43] were used to isolate proteins from the liver for western blot analysis. Briefly, a solution containing 0.1% sodium dodecyl sulfate, 50 mM Tris, 150 mM sodium chloride, 1% sodium deoxycholate, 1% Triton X-100, 1 mM sodium orthovanadate, and 1 mM ethylenediaminetetraacetic acid was used to homogenize liver tissues (Polytron CH-6010; Kinematica GmbH, Luzern, Switzerland). The homogenate was collected and centrifuged at 12,000 rpm at 4 ˚C for 20 min (MX-160 from Tomy Seiko Co., Ltd.). Total cell lysate was preserved in the final supernatant. Then, as previously mentioned [44], western blotting for 5’ adenosine monophosphate-activated protein kinase (AMPK) using primary antibody (Cell Signaling, cat #2532, Danvers, MA), phosphorylated form of α-AMPK (pAMPK) using primary antibody (Cell Signaling, cat #2535, Danvers, MA), Fatty acid synthase (FAS) using primary antibody (Cell Signaling, cat #3180, Danvers, MA), acetyl-CoA carboxylase (ACC) using primary antibody (Cell Signaling, cat #3662, Danvers, MA), and the HRP-linked secondary antibody (Cell Signaling, cat #7074, Danvers, MA) was performed with liver homogenate containing 100 μg protein.
Utilizing the TRIzol RNA isolation reagent (Invitrogen, Carlsbad, CA, USA), total RNA was extracted from liver tissue. According with the manufacturer’s instructions RNA reverse transcription was carried out using the Prime ScriptTM 1st Strand cDNA Synthesis Kit (Takara Bio Inc., Shiga, Japan). Twenty microliters of SYBR® Premix Ex Taq (Takara Bio Inc.) was used for quantitative RT-PCR. The results were adjusted for mRNA signals against β-actin. The primer sequences in Table 1 was used in this study [45].
The means ± standard deviation (SD) of the data are displayed. To perform the statistical analyses using IBM SPSS statistic version 20 (Statistical Package for Social, SPSS Inc., Chicago IL, USA). One-way analysis of variance (ANOVA) was used to assess the data, and Tukey’s multiple comparison test was performed. Differences were deemed statistically significant at p < 0.05.
DPPH radical scavenging analyses were used to assess the antioxidant activities of camellia oil and herb-infused camellia oil, as shown in Figure 1. The DPPH radical scavenging activity was considerably enhanced in a concentration-dependent manner (from 0.625 mg/mL to 10 mg/mL). Moreover, IC50 value (20.05 mg/mL) of herb-infused camellia oil was less than that of pure camellia oil IC50 value (55.17 mg/mL), indicating that the herb-infused oil has a greater scavenging ability than that of camellia oil alone.
We analyzed the in vivo effects of camellia oil and herb-infused camellia oil, the FER, BW, organ weight, and total fat content were analyzed (Table 2). In contrast to the CON group, food consumption, BW gain, and FER were significantly lower in the Cam and Cam+Herb groups after 7 weeks of treatment. Moreover, the kidney, heart, and liver weights were significantly lower in Cam and Cam+Herb groups than in the CON group. Whereas, gastrocnemius muscle weight was not significantly different. Fat content analysis indicated that mesenteric fat, retroperitoneal, epididymal, kidney, and total fat contents were significantly higher in the HFD-induced CON group than in the Cam and Cam+Herb groups.
We analyzed the hepatic and serum markers associated with obesity in mice treated with camellia oil and herb-infused camellia oil. Compared to those of the HFD fed CON group, the Cam and Cam+Herb groups had significantly lower hepatic TC and TG levels, as well as serum TC and TG levels (Table 3). To establish the anti-obesity activity of camellia, we examined blood markers linked to obesity. Compared with those of the NOR, the CON group had higher levels of the marker enzymes AST, ALT, insulin, TNF-α, BUN, LDL cholesterol, and TBARS. However, this increase was dramatically reduced in the Cam and Cam+Herb groups. The adiponectin and HDL cholesterol levels are high in Cam group than in the CON group. However, there were no appreciable differences in blood protein and albumin levels between the four groups.
GPx, GR, GST, and SOD activities and GSH levels were assessed in hepatic homogenates to determine the ability of camellia oil and herb-infused camellia oil to prevent liver impairment in HFD-induced obese mice. As shown in Table 4, the CON group had lower hepatic GSH levels and lower GPx, GR, GST, and SOD enzyme activities than those of the NOR group. GSH levels and antioxidant enzyme activities were both increased by camellia oil treatment after 7 weeks (Cam, Cam+Herb). Moreover, the enzyme activities of the Cam+Herb group were higher than those of the CON and Cam groups.
The histological changes induced by camellia oil and herb-infused camellia oil on the liver and epididymal fat are shown in Figure 2. The mice in the CON group of the HFDfed animals had more severe macrovesicular steatosis than the mice in the NOR group, according to the liver histology findings using H&E staining, as shown in Figure 2(A). Additionally, the liver weight of the CON group increased with strong fat (+++) deposition owing to a significant buildup of hepatic lipid droplets. Moreover, HFD-fed CON group also had a high FER, which contributed to obesity (Table 1). HFD-fed mice are known to grow heavier than mice fed conventional diets are. However, both camellia oil and herb-infused camellia oil (Cam, Cam+Herb) treatments reduced macrovesicular steatosis. Moreover, the deposition of fatty acids decreased to mild (+); in addition, the liver weight decreased significantly compared with the CON group.
Examination of the histological changes in the epididymal tissues showed that the adipocytes of the HFD-fed CON obese group were significantly larger than those of the NOR group which was fed a normal diet [Figure 2(B)]. However, compared to those of the CON group, the adipocytes in the camellia groups (Cam and Cam+Herb) were significantly smaller. Additionally, the total adipocyte area of the CON group was greater (547 μm2) than that of the NOR group (320 μm2). The overall adipocyte area dramatically decreased in both the Cam group (480 μm2) and the Cam+Herb group (426 μm2). These findings demonstrate that treatment with camellia oil and herb-infused camellia oil reduces intracellular lipid accumulation in adipocytes.
Using camellia oil and herb-infused camellia oil, we examined the AMPK and p-AMPK protein expression in HFD-induced obese mice. As shown in Figure 3(A-C), the CON group mice showed that the hepatic AMPK and p-AMPK expression was downregulated. However, these levels dramatically increased in post-treatment with the camellia oil and herbs infused with camellia oil groups. In addition, compared to that in the CON group, the ratio of pAMPK/AMPK levels increased in the Cam and Cam+Herb groups. These findings provide indirect evidence that camellia oil and herb-infused camellia oil exert anti-obesity effects by increasing the levels of AMPK and p-AMPK protein expression in HFD-induced mice.
Additionally, we examined the expression of FAS and ACC in the NOR, CON, and camellia groups (Cam and Cam+Herb). As shown in Figure 4(A, B), the livers of the HFD-induced CON group mice showed higher expression levels of ACC and FAS proteins. Whereas, camellia and herb-infused camellia oil treatment reduced ACC and FAS levels, the herb-infused group more effectively lowered protein expression. These findings suggest that herb-infused camellia oil has a stronger anti-obesity effect than that of camellia oil alone by reducing the production of fatty acids in mice on HFD.
The mRNAs expression in liver is linked to lipogenesis and gluconeogenesis was assessed in HFD-induced obese mice (CON) and camellia oil-treated groups (Cam, Cam+Herb). These findings demonstrate that phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) enzyme expression levels were increased in the CON group, which is consistent with an increase in hepatic glycogen and blood glucose levels. PEPCK and G6Pase RNA expressions was considerably inhibited (p < 0.05) during the 7-week treatment with camellia oil and herb-infused camellia oil, as shown in Figure 5(A, B). However, liver glycogen phosphorylase (LGP) expression increased and glycogen synthase (GS) expression decreased in the HFD-fed CON group, indicating that liver glycogen synthesis was reduced. This further demonstrates the link between fat and insulin resistance, which favors the onset of diabetes. However, after receiving a 7-week treatment with camellia oil and herb-infused camellia oil, GS levels considerably increased, whereas LPG levels declined, as shown in Figure 5(C, D). Additionally, the expression of the lipogenic enzymes ACC, FAS, and stearoyl coenzyme A desaturase (SCD) was enhanced in the CON group, although the hepatic mRNA expression levels in the camellia oil-treated groups (Cam and Cam+Herb) were significantly reduced (Figure 5(E-G). Compared to those in the NOR, the CON group had lower levels of carnitine palmitoyltransferase (CPT) gene expression, which is associated with fatty acid oxidation. Figure 5(H) shows that the decline in CPT expression levels was substantially higher in the groups that received camellia treatment. Additionally, Figure 5(I) shows that pyruvate dehydrogenase kinase (PDK) gene expression was increased in the CON group, whereas it was significantly reduced in the camellia oil-treated groups (Cam, Cam+Herb).
Camellia oil, an edible oil with very high nutritional value, is rich in unsaturated fatty acids and phytochemicals such as squalene, sterols, tocopherols, and polyphenols [46]. These compounds also possess a variety of bioactivities, including antioxidant, anticancer, and anti-inflammatory properties [47]. Moreover, herbs (rosemary and cloves) are rich in phytochemicals, such as alkaloids, tannins, flavonoids, and phenols, which are responsible for some of their biochemical characteristics. Owing to its economic advantages, camellia oil is universally recognized as a natural source of antioxidants. It contains several organic compounds and exhibits improved radical-scavenging performance in the DPPH and ABTS (2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)) assays [48]. In this study, camellia oil (Cam) and herb-infused camellia oil (Cam+Herb) showed lower IC50 values, indicating a stronger capacity to scavenge DPPH radicals. Additionally, compared to those observed in the camellia oil-treated group, the herb-infused camellia oil treatment had a stronger scavenging ability and lower IC50 value. The antioxidant properties of the phenolic compounds tannins, flavan-3-ols, catechol, and pyrogallol in camellia oil were assessed by Gao et al. [49], who concluded that they have considerable DPPH radical scavenging activity. According to previous reports, the scavenging of hydroxyl radicals by flavanols and catechins, including camellia oil, have shown that the amount of hydroxyl radicals affects how organic matter reacts [50]. Further, the present experimental findings show that the pretreatment of cells with camellia oil can lower ROS generation.
In numerous strains of mice and rats, high-fat foods have been shown to increase body weight and cause diabetes. C57BL/6J mice, which develop obesity, hyperinsulinemia, hyperglycemia, and hypertension when fed a HFD ad libitum, are a particularly effective model for simulating human metabolic abnormalities observed in obesity [51]. This is primarily caused by an increase in the consumption of foods that are high in calories and have high levels of sugar and saturated fats (HFD), along with a decrease in physical activity. The increase in size and quantity of adipocytes in adipose tissues are considered to have a cellular syndrome of obesity, and the increase in adipocyte size is due to increased lipid cellular storage [52]. According to Avtanski et al. [53], male C57BL/6 mice are characterized by excess body weight, fat deposits, liver accumulation, and inflammation after receiving HFD for 9 weeks. The current study, we examined the anti-obesity effects of camellia oil and herb-infused camellia oil in HFD-fed mice. Camellia treatment reduced body weight gain by lowering the mass of the liver and visceral white adipose tissue and marginally increasing insulin sensitivity in HFD-fed mice. Moreover, our data revealed that the HFD-fed mice had higher hepatic TC and TG levels. This indicated that the HFD diet led to excess fat accumulation in the liver tissue compared to that in the camellia-treated groups. It indicated that camellia treatment has a beneficial effect in reducing aberrant fat accumulation in the hepatic tissue. The liver weight of the HFD-induced CON group was not substantially different from that of the NOR group because HFD-induced mice may eat less as their appetite is reduced [54]; however, the hepatic TC and TG levels were significantly higher than those of the normal (NOR) and camellia-treated groups (Cam and Cam+Herbs) due to excessive fat accumulation in HFD-induced mice. Nagao et al. [55] investigated how LDL cholesterol, body weight, and fat mass, especially the visceral fat region, could be reduced in humans by treatment with catechin-containing camellia oil. Li et al. [56] investigated the effects of camellia and found that the plant’s primary chemical constituents had dose-dependent anti-adipogenic activity and significantly reduced the accumulation of lipid droplets in liver and fat tissues.
Histological analysis of the liver tissues of CON group revealed vacuole development with fat accumulation, hepatocellular ballooning, and microvesicular steatosis. In mice belonging to the CON group, fatty liver was a result of increased levels of metabolic indicators, such as TC, AST, ALT, and TG [57]. In addition, HFD-induced control obese mice (CON group) also had higher body weights than those of normal mice, along with an adipose tissue weight increase caused by deposition of fat including larger adipocytes. Moreover, the blood levels of adiponectin, TNF-α, TBARS, and LDL cholesterol are thought to be the main contributors to chronic inflammation linked to obesity [58-62]. Camellia oil and herb-infused camellia oil gradually decreased inflammatory vesicular steatosis. In addition, the anti-obesity effect of camellia oil decreased the serum levels of adiponectin, TNF-α, TBARS, and LDL cholesterol. Further, the LDL cholesterol level in the Cam+Herb group decreased more significantly than that in the Cam group. These findings imply that herb-infused camellia oil has more anti-obesity effects than those of camellia oil alone in the context of the fatty liver.
Obese mice in the HFD-induced control group (CON) have a pro-oxidant condition due to their visceral adipose tissue and liver can generates the pro-inflammatory cytokines [63] and have lower antioxidant enzyme activity [64]. According to the results of the present study, HFD-induced obesity in CON mice led to increased oxidative stress and inflammation in the liver, which in turn decreased the activity of antioxidant enzymes such GPx, GR, GST, and SOD as well as GSH levels. This decline in antioxidant enzyme activity may be caused by the rapid and exhausting use of antioxidant enzymes that have been stockpiled to fight free radical production in obesity; obesity-associated oxidative stress-involves processes that can enhance fat deposition in organs and free radical production [65]. In addition, high levels of lipid peroxidation, hypertension, and hypercholesterolemia are plausible causes of elevated oxidative stress in obesity [66]. The antioxidant enzyme activities increased in HFDfed mice upon treatment with camellia oil and herbs-infused camellia oil. Additionally, herbs-infused oil treatment more efficiently boosts antioxidant activities than the effects of camellia oil treatment [67]. The reduction of hepatic antioxidant enzyme activity was successfully restored by herbs-infused camellia treatment in HFD-induced obese CON mice, indicating that herb-infused camellia oil treatment is advantageous for increasing antioxidant effects in obese mice.
Owing to the rising prevalence of pathogenic metabolic diseases, AMPK activity is dysregulated in obese mice [68]. Obesity and high insulin resistance correlate with reduced AMPK activation [69,70]. Our findings revealed that the HFD-induced obese CON group exhibited lower AMPK and p-AMPK protein expression. According to the dysregulation of AMPK signaling or the absence of AMPK expression in obese mice, lower AMPK activity is associated with the etiology of obesity [71]. In HFD-induced obese mice, camellia oil and herb-infused camellia oil treatment activated AMPK and phosphorylated AMPK (p-AMPK) and decreased ACC, thereby promoting fatty acid oxidation [72]. p-AMPK is an essential protein that controls lipid and carbohydrate metabolism [73].
An important enzyme for the production of long-chain fatty acids is ACC, along with FAS, it can control the pace of hepatic triglyceride synthesis. According to the current findings, obese CON group that were fed an HFD had higher levels of FAS and ACC than those of the NOR group. As a result, it plays a significant role in the onset of fatty liver [74]. Elevated hepatic FAS and ACC levels are mostly caused by high insulin levels [75]. Additionally, the HFD-fed CON group displayed elevated FAS and ACC expression without promoting liver phosphorylation. Following treatment with camellia oil and herb-infused camellia oil, the increased FAS and ACC levels were reduced. According to these findings, treatment with herb-infused camellia oil lowered the levels of FAS and ACC and liver steatosis by activating AMPK and p-AMPK.
Increased expression of lipogenic enzymes (FAS, ACC, and SCD), gluconeogenic enzymes (PEPCK and G6Pase), and the AMPK protein expression may assist to explain the elevated liver weight, including hepatic steatosis, in HFD-induced obese CON mice. The hepatic gluconeogenic enzymes expressions can cause chronic hepatic glucose production, hyperglycemia, and insulin resistance in obese and diabetic animals [76]. Our findings showed that PEPCK and G6Pase mRNA levels increased in the HFD-induced obese CON group, indicating that the HFD-fed mice produced excess glucose. To maintain glucose homeostasis, these levels were reduced in the liver tissues of the camellia oil (Cam) and herb-infused camellia oil (Cam+Herb) groups.
The activities of GS and LGP regulate the ability of the liver to synthesize glycogen. In the context of lipid accumulation, glycogen production is reduced concurrently with declining expression of GS [77]. These findings suggest that lowering AMPK levels, fat storage, and hepatic steatosis occurred in obese mice (CON) by increasing the expression of mRNAs encoding the lipogenic enzymes ACC, FAS, and SCD. The use of camellia oil and herb-infused camellia oil may stimulate phosphorylated AMPK signaling and prevent the mRNA expression of the transcription factors ACC, FAS, and SCD, resulting in decreased mRNA transcription and downregulation of enzymatic activity [78,79]. This suggests that camellia and herb-infused camellia oils inhibit lipogenesis and successfully prevent hepatic steatosis in HFD-induced obese mice. When the mRNA expression of CPT is suppressed, there is a deficit in fatty acid oxidation, which results in fatty liver [80]. In contrast, obese mice show higher levels of PDK mRNA expression [81]. However, treatment with camellia and herb-infused camellia oils improved CPT mRNA expression and protected against fat accumulation in liver tissues.
In conclusion, our findings showed that camellia and herb-infused camellia oil have the strongest capacity to scavenge free radicals. Through the AMPK pathway in the liver and adipocytes, these positive effects may be modulated by the lipid profile and a decrease in the expression levels of lipogenic enzymes such as FAS and ACC. In addition to reducing body weight, liver steatosis, and the formation of TG and lipids in the liver and adipose tissues, the herb-infused camellia oil treatment provided a stronger anti-obesity effect than that of camellia oil treatment. This suggests that treatment with the herb-infused camellia oil has a positive effect on diabetes-induced obesity in rats.
Acknowledgments
This research was supported by the Starting Growth Technological R&D Program funded by the Ministry of SMEs and Startups (MSS, Korea) (S3308965).
Figure 1.
DPPH radicals scavenging activity (%) of camellia oil (Cam) and herb-infused camellia oil (Cam+Herb). All values are mean ± SD. Values that do not share a common letter are substantially different at p < 0.05 when applying Tukey’s multiple comparison test.
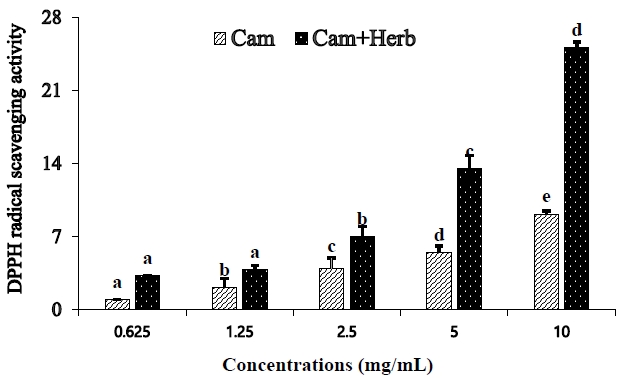
Figure 2.
Effect of camellia oil and herb-infused camellia oil on the histology of mouse tissues (A) liver fat contents, and (B) epididymal fat adipocyte area in NOR, CON, Cam, and Cam+Herb groups.
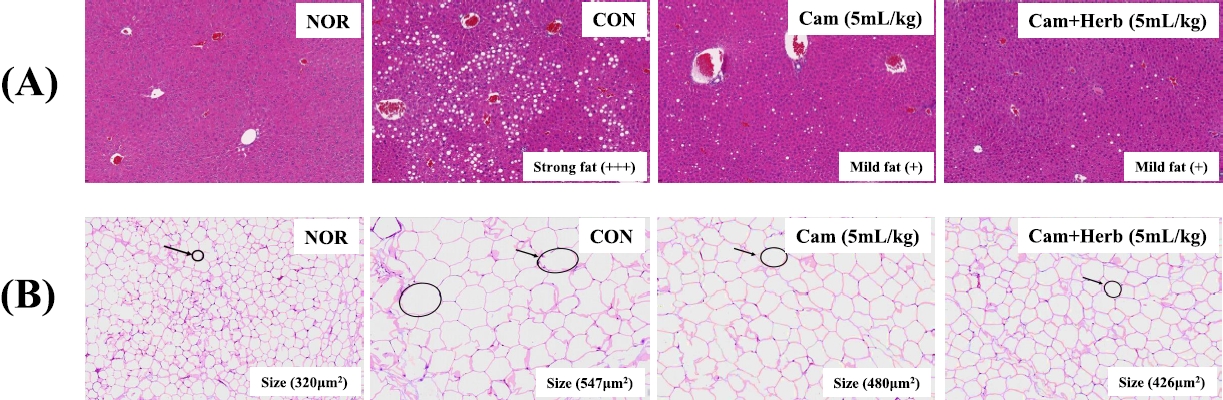
Figure 3.
Effect of camellia oil and herb-infused camellia oil on the (A) expression of AMPK and p-AMPK in liver tissue is analyzed by western blotting using antibodies against AMPK and p-AMPK, (B) Protein bands are quantified by ImageJ. β-actin is used as control to evaluate relative expression of protein, (C) pAMPK/AMPK ratio. Each statistic is the mean ± standard deviation (SD) (n=4). Values that do not share a common letter are substantially different at p < 0.05 when applying Tukey’s multiple comparison test.

Figure 4.
Effect of camellia oil and herb-infused camellia oil on the (A) expression of FAS and ACC in liver tissue are analyzed by western blot, (B) Protein bands are quantified by ImageJ. β-actin is used as control to evaluate relative expression of protein. Each statistic is the mean ± standard deviation (SD) (n=4). Values that do not share a common letter are substantially different at p < 0.05 when applying Tukey’s multiple comparison test.
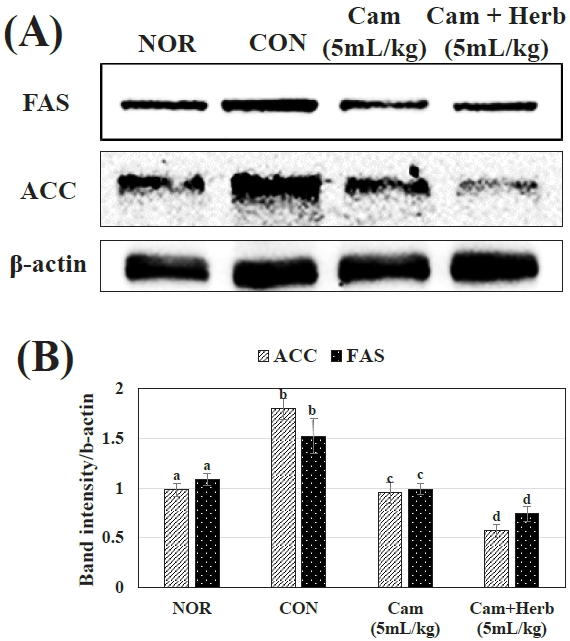
Figure 5.
Effect of camellia oil and herb-infused camellia oil on the mRNA expression of glucose and lipid metabolism related genes. (A) phosphoenolpyruvate carboxykinase (PEPCK), (B) glucose-6-phosphatase (G6Pase), (C) glycogen synthase (GS), (D) liver glycogen phosphorylase (LGP), (E) fatty acid synthase (FAS), (F) acetyl-CoA carboxylase (ACC), (G) stearoyl coenzyme A desaturase (SCD), (H) pyruvate dehydrogenase kinase (PDK) and fatty acid oxidation related genes (I) carnitine palmitoyltransferase (CPT) in the liver. Each statistic is the mean ± standard deviation (SD) (n=6). Values that do not share a common letter are substantially different at p < 0.05 when applying Tukey’s multiple comparison test.

Table 1.
A list of the primers that were utilized in this work to analyze gene expression.
Table 2.
Initial body weight, food efficiency ratio (FER) and organs weight including different region of fat content in standard diet given NOR group and high fat diet (HFD) treated CON, Cam, and Cam+Herb groups C57 BL/6J mice.
Table 3.
Effects of camellia oil (Cam) and herbs infused camellia oil (Cam+Herb) on hepatic and serum biochemical parameters in high fat diet treated mice.
Table 4.
Effects of camellia oil (Cam) and herbs infused camellia oil (Cam+Herb) on hepatic antioxidant enzymes in NOR, CON, Cam and Cam+Herb treated mice.
Glutathione peroxidase (GPx), glutathione reductase (GR), glutathione S-transferase (GST), superoxide dismutase (SOD) and glutathione (GSH). Each statistic is the mean ± standard deviation (SD) (n=6). Values that do not share a common letter are substantially different at p < 0.05 when applying Tukey’s multiple comparison test.
REFERENCES
1. Bray GA, Kim KK, Wilding JPH; World Obesity Federation. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev 2017;18:715-23.



4. Volta F, Gerdes JM. The role of primary cilia in obesity and diabetes. Ann NY Acad Sci 2017;1391:71-84.



5. Xu T, Sheng Z, Yao L. Obesity-related glomerulopathy: pathogenesis, pathologic, clinical characteristics, and treatment. Front Med 2017;11:340-8.



7. Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol Life Sci 2018;75:3313-27.




8. Liu Q, Bengmark S, Qu S. The role of hepatic fat accumulation in pathogenesis of non-alcoholic fatty liver disease (NAFLD). Lipids Health Dis 2010;9:42.




9. Jager J, Aparicio‐Vergara M, Aouadi M. Liver innate immune cells and insulin resistance: the multiple facets of Kupffer cells. J Intern Med 2016;280:209-20.



10. Li Y, Wu J, Cao C, Zhu X, Sun X, Wu R. Effects of skim milk fermented with Lactobacillus plantarum WW on the constitutions of rats fed a high-fat diet. J Dairy Sci 2020;103:5019-29.


11. Chu DT, Malinowska E, Jura M, Kozak LP. C57BL/6J mice as a polygenic developmental model of diet‐induced obesity. Physiol Rep 2017;5:e13093.




12. Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008;57:1470-81.



13. Daniel H, Gholami AM, Berry D, Desmarchelier C, Hahne H, Loh G, Mondot S, Lepage P, Rothballer M, Walker A, Bohm C, Wenning M, Wagner M, Blaut M, Schmitt-Kopplin P, Kuster B, Haller D, Clavel T. High-fat diet alters gut microbiota physiology in mice. ISME J 2014;8:295-308.



14. Talukdar S, Oh DY, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y, Ofrecio J, Lin M, Brenner MB, Olefsky JM. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med 2012;18:1407-12.




15. Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab 2004;89:463-78.


16. Clapham JC, Arch JRS. Thermogenic and metabolic antiobesity drugs: rationale and opportunities. Diabetes Obes Metab 2007;9:259-75.


17. Konstantinidi M, Koutelidakis AE. Functional foods and bioactive compounds: a review of its possible role on weight management and obesity’s metabolic consequences. Medicines 2019;6:94.



18. Luan F, Zeng J, Yang Y, He X, Wang B, Gao Y, Zeng N. Recent advances in Camellia oleifera Abel: a review of nutritional constituents, biofunctional properties, and potential industrial applications. J Funct Foods 2020;75:104242.

19. Huang T, Jiang J, Cao Y, Huang J, Zhang F, Cui G. Camellia oil (Camellia oleifera Abel.) treatment improves high-fat diet-induced atherosclerosis in apolipoprotein E (ApoE)−/− mice. Biosci Microbiota Food Health 2023;42:56-64.

20. Tu PS, Tung YT, Lee WT, Yen GC. Protective effect of camellia oil (Camellia oleifera Abel.) against ethanol-induced acute oxidative injury of the gastric mucosa in mice. J Agric Food Chem 2017;65:4932-41.


22. Wang S, Moustaid-Moussa N, Chen L, Mo H, Shastri A, Su R, Bapat P, Kwun I, Shen CL. Novel insights of dietary polyphenols and obesity. J Nutr Biochem 2014;25:1-18.



23. Bumrungpert A, Pavadhgul P, Kalpravidh RW. Camellia oil-enriched diet attenuates oxidative stress and inflammatory markers in hypercholesterolemic subjects. J Med Food 2016;19:895-8.


24. Shen TT, Wu SX. Effects of tea seed oil on hyperlipidemic rats induced by high-fat diet. Food Sci Technol Res 2017;23:101-9.

25. Huang T, Zhou W, Ma X, Jiang J, Zhang F, Zhou W, He H, Cui G. Oral administration of camellia oil ameliorates obesity and modifies the gut microbiota composition in mice fed a high-fat diet. FEMS Microbiol Lett 2021;368:fnab063.



26. Xiao X, He L, Chen Y, Wu L, Wang L, Liu Z. Anti-inflammatory and antioxidative effects of Camellia oleifera Abel components. Future Med Chem 2017;9:2069-79.


27. Wu X, Huang Y, Xie Z. Health functions and prospective of Camellia oil. Food Sci Technol 2005;8:94-6.
28. Sedighi R, Zhao Y, Yerke A, Sang S. Preventive and protective properties of rosemary (Rosmarinus officinalis L.) in obesity and diabetes mellitus of metabolic disorders: a brief review. Curr Opin Food Sci 2015;2:58-70.

29. Yu MH, Choi JH, Chae IG, Im HG, Yang SA, More K, Lee IS, Lee J. Suppression of LPS-induced inflammatory activities by Rosmarinus officinalis L. Food chem 2013;136:1047-54.


30. Romo‐Vaquero M, Larrosa M, Yáñez‐Gascón MJ, Issaly N, Flanagan J, Roller M, Tomás‐Barberán FA, Espín JC, García‐ Conesa MT. A rosemary extract enriched in carnosic acid improves circulating adipocytokines and modulates key metabolic sensors in lean Zucker rats: critical and contrasting differences in the obese genotype. Mol Nutr Food Res 2014;58:942-53.



31. Romo Vaquero M, Yáñez-Gascón MJ, Garcia Villalba R, Larrosa M, Fromentin E, Ibarra A, Roller M, Tomás-Barberán F, Espín de Gea JC, García-Conesa MT. Inhibition of gastric lipase as a mechanism for body weight and plasma lipids reduction in Zucker rats fed a rosemary extract rich in carnosic acid. PLoS One 2012;7:e39773.



32. Wang QL, Li H, Li XX, Cui CY, Wang R, Yu NX, Chen LX. Acute and 30-day oral toxicity studies of administered carnosic acid. Food Chem Toxicol 2012;50:4348-55.


33. Prasad RC, Herzog B, Boone B, Sims L, Waltner-Law M. An extract of Syzygium aromaticum represses genes encoding hepatic gluconeogenic enzymes. J Ethnopharmacol 2005;96:295-301.


34. Mejía-Argueta E, Santillán-Benítez J, Canales-Martinez M, Mendoza-Medellín A. Antimicrobial activity of Syzygium aromaticum L. essential oil on extended-spectrum beta-lactamases-producing Escherichia coli. Bull Natl Res Cent 2020;44:201.
35. Ahmad S, Latif A, Qasmi IA. Effect of 50% ethanolic extract of Syzygium aromaticum (L.) Merr. & Perry.(clove) on sexual behaviour of normal male rats. BMC Complement Alter Med 2004;4:17.
36. Zhu Z, Liang H, Sun DW. Infusing silicone and Camellia seed oils into micro-/nanostructures for developing novel anti-icing/frosting surfaces for food freezing applications. ACS Appl Mater Interfaces 2023;15:14874-83.




37. Ko W, Lee H, Kim N, Jo HG, Woo ER, Lee K, Han YS, Park SR, Ahn G, Cheong SH, Lee DS. The anti-oxidative and anti-neuroinflammatory effects of sargassum horneri by heme oxygenase-1 induction in BV2 and HT22 cells. Antioxidants 2021;10:859.



38. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351-8.


39. Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 1957;28:56-63.


40. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248-54.


41. Park SY, Fernando IPS, Han EJ, Kim MJ, Jung K, Kang DS, Ahn CB, Ahn G. In vivo hepatoprotective effects of a peptide fraction from krill protein hydrolysates against alcohol-induced oxidative damage. Mar Drugs 2019;17:690.



42. Kim KN, Kang MC, Kang N, Kim SY, Hyun CG, Roh SW, Ko EY, Cho K, Jung WK, Ahn G, Jeon YJ, Kim D. 2, 4, 6-Trihydroxybenzaldehyde, a potential anti-obesity treatment, suppressed adipocyte differentiation in 3T3-L1 cells and fat accumulation induced by high-fat diet in C57BL/6 mice. Environ Toxicol Pharmacol 2015;39:962-8.


43. Arunkumar E, Anuradha CV. Genistein promotes insulin action through adenosine monophosphate-activated protein kinase activation and p70 ribosomal protein S6 kinase 1 inhibition in the skeletal muscle of mice fed a high energy diet. Nutr Res 2012;32:617-25.


44. Minakawa M, Miura Y, Yagasaki K. Piceatannol, a resveratrol derivative, promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in L6 myocytes and suppresses blood glucose levels in type 2 diabetic model db/db mice. Biochem Biophys Res Commun 2012;422:469-75.


45. Wang Y, Nair S, Gagnon J. Herring milt and herring milt protein hydrolysate are equally effective in improving insulin sensitivity and pancreatic beta-cell function in diet-induced obese-and insulin-resistant mice. Mar Drugs 2020;18:635.



46. Chang M, Qiu F, Lan N, Zhang T, Guo X, Jin Q, Liu R, Wang X. Analysis of phytochemical composition of Camellia oleifera oil and evaluation of its anti-inflammatory effect in lipopolysaccharide- stimulated RAW 264.7 macrophages. Lipids 2020;55:353-63.



47. Xiao X, He L, Chen Y, Wu L, Wang L, Liu Z. Anti-inflammatory and antioxidative effects of Camellia oleifera Abel components. Future Med Chem 2017;9:2069-79.


48. Lee CP, Yen GC. Antioxidant activity and bioactive compounds of tea seed (Camellia oleifera Abel.) oil. J Agric Food Chem 2006;54:779-84.


49. Gao DF, Zhang YJ, Yang CR, Chen KK, Jiang HJ. Phenolic antioxidants from green tea produced from Camellia taliensis. J Agric Food Chem 2008;56:7517-21.


50. Symons MCR, Gutteridge JMC. Free Radicals and Iron: Chemistry, Biology, and Medicine. Oxford: Oxford Science Publications. 1998.
51. Wang CY, Liao JK. A mouse model of diet-induced obesity and insulin resistance. Methods Mol Biol 2012;821:421-33.



52. Hirsch J, Han PW. Cellularity of rat adipose tissue: effects of growth, starvation, and obesity. J Lipid Res 1969;10:77-82.


53. Avtanski D, Garcia A, Caraballo B, Thangeswaran P, Marin S, Bianco J, Lavi A, Poretsky L. In vitro effects of resistin on epithelial to mesenchymal transition (EMT) in MCF-7 and MDA-MB-231 breast cancer cells-qRT-PCR and Westen blot analyses data. Data Brief 2019;25:104118.



54. Grancieri M, Martino HSD, de Mejia EG. Chia seed (Salvia hispanica L.) as a source of proteins and bioactive peptides with health benefits: a review. Compr Rev Food Sci Food Saf 2019;18:480-99.



55. Nagao T, Hase T, Tokimitsu I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity 2007;15:1473-83.


56. Li KK, Wong HL, Hu T, Zhang C, Han XQ, Ye CX, Leung PC, Cheng BH, Ko CH. Impacts of Camellia kucha and its main chemical components on the lipid accumulation in 3T3‐L1 adipocytes. Int J Food Sci Technol 2016;51:2546-55.


57. Han JM, Kim HI, Lee Y-J, Lee JW, Kim KM, Bae JC. Differing associations between fatty liver and dyslipidemia according to the degree of hepatic steatosis in Korea. J Lipid Atheroscler 2019;8:258-66.




58. Choi HM, Doss HM, Kim KS. Multifaceted physiological roles of adiponectin in inflammation and diseases. Int J Mol Sci 2020;21:1219.



59. Kizer JR, Arnold AM, Strotmeyer ES, Ives DG, Cushman M, Ding J, Kritchevsky SB, Chaves PH, Hirsch CH, Newman AB. Change in circulating adiponectin in advanced old age: determinants and impact on physical function and mortality. The Cardiovascular Health Study All Stars Study. J Gerontol A Biol Sci Med Sci 2010;65:1208-14.


60. Morin CL, Gayles EC, Podolin DA, Wei Y, Xu M, Pagliassotti MJ. Adipose tissue-derived tumor necrosis factor activity correlates with fat cell size but not insulin action in aging rats. Endocrinology 1998;139:4998-5005.

61. Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem 2017;524:13-30.


62. Ghani MA, Barril C, Bedgood Jr DR, Prenzler PD. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem 2017;230:195-207.


63. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004;89:2548-56.


64. Marseglia L, Manti S, D’Angelo G, Nicotera A, Parisi E, Di Rosa G, Gitto E, Arrigo T. Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci 2014;16:378-400.



65. Vincent HK, Powers SK, Dirks AJ, Scarpace PJ. Mechanism for obesity-induced increase in myocardial lipid peroxidation. Int J Obes 2001;25:378-88.


66. Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes 2006;30:400-18.


67. Wang M, YU B, He J, Yu J, Luo YH, Luo JQ, Mao XB, Chen DW. The toxicological effect of dietary excess of saccharicterpenin, the extract of camellia seed meal, in piglets. J Integr Agric 2020;19:211-24.

68. Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov 2004;3:340-51.



69. Kelly M, Keller C, Avilucea PR, Keller P, Luo Z, Xiang X, Giralt M, Hidalgo J, Saha AK, Pedersen BK, Ruderman NB. AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise. Biochem Biophys Res Commun 2004;320:449-54.


70. Yu X, McCorkle S, Wang M, Lee Y, Li J, Saha AK, Unger RH, Ruderman NB. Leptinomimetic effects of the AMP kinase activator AICAR in leptin-resistant rats: prevention of diabetes and ectopic lipid deposition. Diabetologia 2004;47:2012-21.



71. Wu Y, Song P, Xu J, Zhang M, Zou MH. Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J Biol Chem 2007;282:9777-88.


72. Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen ZP, Witters LA. Dealing with energy demand: the AMP-activated protein kinase. Trends Biochem Sci 1999;24:22-5.


73. Bijland S, Mancini SJ, Salt IP. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin Sci 2013;124:491-507.


74. Wiegman CH, Bandsma RH, Ouwens M, van der Sluijs FH, Havinga R, Boer T, Reijngoud DJ, Romijn JA, Kuipers F. Hepatic VLDL production in ob/ob mice is not stimulated by massive de novo lipogenesis but is less sensitive to the suppressive effects of insulin. Diabetes 2003;52:1081-9.



75. Shillabeer G, Hornford J, Forden JM, Wong NC, Russell JC, Lau DC. Fatty acid synthase and adipsin mRNA levels in obese and lean JCR: LA-cp rats: effect of diet. J Lipid Res 1992;33:31-9.


76. Liu Y, Nakagawa Y, Wang Y, Sakurai R, Tripathi PV, Lutfy K, Friedman TC. Increased glucocorticoid receptor and 11β-hydroxysteroid dehydrogenase type 1 expression in hepatocytes may contribute to the phenotype of type 2 diabetes in db/db mice. Diabetes 2005;54:32-40.



77. Parker GJ, Lund KC, Taylor RP, McClain DA. Insulin resistance of glycogen synthase mediated byo-linked N-acetylglucosamine. J Biol Chem 2003;278:10022-7.


78. Hong M, Li N, Li J, Li W, Liang L, Li Q, Wang R, Shi H, Storey KB, Ding L. Adenosine monophosphate-activated protein kinase signaling regulates lipid metabolism in response to salinity stress in the red-eared slider turtle Trachemys scripta elegans. Front Physiol 2019;10:962.



79. Miyazaki M, Dobrzyn A, Man WC, Chu K, Sampath H, Kim HJ, Ntambi JM. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and-independent mechanisms. J Biol Chem 2004;279:25164-71.


-
METRICS

-
- 1 Crossref
- Scopus
- 1,319 View
- 43 Download
- Related articles in Phys Act Nutr





