Four weeks of lower-limb static stretching reduces regional arterial stiffness in middle-aged and older women
Article information
Abstract
[Purpose]
This study aimed to clarify whether habitual lower-limb stretching intervention reduces regional arterial stiffness at the stretched site in middle-aged and older women.
[Methods]
In this study, the effects of 4 weeks of lower-limb static stretching (of the hip extensor and flexor, knee extensor and flexor, and plantar flexor muscles) were investigated on systemic, central, and peripheral arterial stiffness using pulse wave velocity in 14 healthy middle-aged and older women randomly assigned to either a sedentary control group (67.3 ± 5.6 years; n = 7) or a stretching intervention group (63.4 ± 6.4 years; n = 7).
[Results]
The femoral-ankle pulse wave velocity (an index of peripheral arterial stiffness) significantly decreased in the intervention group (pre, 1222.4 ± 167.5 cm/s; post, 1122.0 ± 141.1 cm/s) but did not change in the control group (pre, 1122.7 ± 107.7 cm/s; post, 1139.9 ± 77.5 cm/s). However, the brachial-ankle pulse wave velocity as an index of systemic arterial stiffness (control: pre, 1655.7 ± 296.8 cm/s, post, 1646.4 ± 232.1 cm/s; intervention: pre, 1637.6 ± 259.9 cm/s, post, 1560.8 ± 254.7 cm/s) and the carotid-femoral pulse wave velocity as an index of central arterial stiffness (control: pre, 1253.6 ± 346.4 cm/s, post, 1223.6 ± 263.4 cm/s; intervention: pre, 1125.4 ± 204.7 cm/s, post, 1024.9 ± 164.5 cm/s) did not change in either group.
[Conclusion]
These findings suggest that lower-limb stretching interventions may reduce regional arterial stiffness at the stretched site
INTRODUCTION
Arterial stiffness is well recognized as an increased risk factor for cardiovascular disease and has been shown to be a predictor of cardiovascular events and mortality [1-3]. Both central and peripheral arterial stiffness increase with age [4]. Habitual aerobic exercises, such as walking and jogging, reduce arterial stiffness [5,6]. However, it is difficult for middle-aged and older people to continuously perform aerobic exercise, which places a heavy burden on the knees and lower calf [7,8]. Recently, to avoid the fear of exposure to coronavirus disease 2019, it has become necessary to propose an exercise mode for middle-aged and older people that can be easily performed alone in a limited space [9,10].
Traditionally, static stretching has been used to improve the flexibility of skeletal muscles and tendons and the range of motion of joints. Several previous studies have shown that habitual static stretching in the whole body, including the upper and lower limbs, neck, and trunk, reduces systemic arterial stiffness [11-13]. Notably, our recent studies demonstrated that acute passive one-legged calf static stretching reduced arterial stiffness only in the lower-limb arteries of the stretched leg, but not in the nonstretched leg [14], owing to a transient increase in shear rate via blood flow in the stretched leg [15]. Thus, the static stretching may lead to a reduction in arterial stiffness only in the stretched region. On the other hand, another study showed that one- or both-legged thigh static stretching for 12 weeks reduced arterial stiffness in the stretched as well as non-stretched leg and upper limb [16]. Taken together, the effects of habitual lower-limb static stretching on systemic, central, and peripheral arterial stiffness remain unclear.
This study aimed to determine whether habitual lower-limb stretching reduced only lower-limb arterial stiffness but not central arterial stiffness. We assessed systemic, central, and regional arterial stiffness before and after a 4-week static stretching intervention in middle-aged and older women using pulse wave velocity (PWV), which is a simple measure of the time taken by the pressure wave to travel over a specific distance and the most widely used gold standard measure of arterial stiffness.
METHODS
Subjects
Fourteen middle-aged and older women (age, 65.3 ± 6.3 years; height, 157.5 ± 5.3 cm; body weight, 53.3 ± 6.1 kg; lean body mass, 35.1 ± 3.1 kg; body fat, 30.2 ± 4.1 %) participated in this study. Participants were recruited through flyers and posters placed in the local community. None of the participants habitually exercised (e.g., aerobic and/or resistance training), had cardiovascular and/or orthopedic diseases, were smokers, or used prescriptions or over-thecounter medications. All participants in this study were postmenopausal for at least 5 years. The participants were randomly assigned to a control group (n = 7) or a stretching intervention group (n = 7), without stratification by parameters such as age or blood pressure. All participants provided written informed consent to participate in the study, which was approved by the ethics committee of Ritsumeikan University and conducted in accordance with the Declaration of Helsinki.
Experimental design
On the test days, all participants refrained from caffeine, alcohol, and strenuous physical activity for 24 h, which was followed by a 6 h fast (water could be consumed freely during the fast). Before the participants were tested, they sat quietly for at least 15 min. Height, body weight (Inner-Scan50V BC-622; Tanita, Tokyo, Japan), PWVs, systolic and diastolic blood pressures, and heart rates (form PWV/ ABI; Omron Colin, Kyoto, Japan) were measured. All measurements were performed before and four weeks after the intervention.
Stretching intervention
In this study, each stretching session during the intervention was performed twice, in the morning and evening, for approximately 15 min, 4 days per week, for 4 weeks. The stretching session included five exercises (Figure 1) for the hip flexor, knee extensor, knee flexor, plantar flexor, and hip extensor muscles: 30 s at the end range (point of minimal discomfort), followed by a 10 s relaxation period twice on both legs in alternate shifts. All participants were asked not to change their physical activity or dietary intake during the study period. All participants in the intervention group were instructed on the stretching method after the baseline measurements, and subsequent stretches were performed at home. The dates of the stretching interventions were recorded on a recording sheet by the participants. The participants in the intervention group performed stretching for 6.3 ± 0.9 days/week (range, 4–7 days/week).
Images and schedule of five static stretching exercises
Panel A, hip flexor muscle; Panel B, knee extensor muscle; Panel C, knee flexor muscle; Panel D, plantar flexor muscle; Panel E, hip extensor muscle. Each stretching exercise was performed on both legs in alternate shifts for 30 seconds at the end range (point of minimal discomfort) followed by a 10 second relaxation period. It was repeated two times a day i.e. morning and evening for 4 days/week for four weeks consecutively.
Measurements of PWV, blood pressure, and heart rate
Carotid and femoral arterial pulse waves, bilateral brachial and ankle blood pressures, and heart rate were assessed using a vascular testing device form PWV/ABI as previously described [14]. The cuffs were wrapped around both the arm and ankle, and the brachial and posterior tibial arterial pressure waveforms were observed using oscillometric pressure sensors. Carotid and femoral arterial pressure waveforms were obtained by placing an applanation tonometry sensor on the left common carotid and femoral arteries. The distance from the left carotid artery to the left femoral artery was measured using a nonelastic measurement tape along a straight line. The distances between the femoral-ankle and brachial-ankle positions were automatically calculated using the formulas [(0.2486 × height + 30.709)] and [(0.5643 × height − 18.381) + (0.2486 × height + 30.709) − (0.2195 × height − 2.0734)], respectively [17,18]. Electrocardiogram electrodes were fixed on both wrists, and a microphone was positioned on the left edge of the sternum to measure heart rate. PWV was calculated as the distance between two arterial measuring sites divided by the transit time. The carotid, femoral, brachial, and ankle arterial waveforms were simultaneously recorded by the sensor, and the transient time was automatically calculated by relating the foot of each waveform to the R-wave of the electrocardiogram. The carotid-femoral PWV (cfPWV) represents central arterial stiffness. Furthermore, the femoral-ankle PWV (faPWV), an index of peripheral arterial stiffness, and brachial-ankle PWV (baPWV), an index of whole-body arterial stiffness including central and peripheral arteries, were measured. The mean values of the right and left baPWV, faPWV, brachial and ankle systolic blood pressure, and diastolic blood pressure were calculated for analysis.
Statistical analysis
Values are expressed as mean ± SD. Two-way (time × condition) repeated-measures ANOVA was performed to determine the changes in body weight, lean body mass, body fat, baPWV, cfPWV, faPWV, blood pressure, and heart rate. Fisher’s post-hoc test was applied when the measurements of each condition were significantly different. An unpaired t-test was performed to determine the differences in the amount of baPWV [post-pre], cfPWV [post-pre], and faPWV [post-pre]. Statistical significance was set at P < 0.05. All statistical analyses were performed using StatView 5.0 (SAS Institute, Tokyo, Japan).
RESULTS
Participant characteristics
At baseline, no significant differences were observed between the groups in terms of age, height, body weight, lean body mass, or body fat (Table 1). The changes in body weight, lean body mass, and body fat after intervention did not differ between the groups (Table 1). There were no significant differences observed in blood pressure and heart rate after the intervention in either group (Table 1).
Response to PWVs before and after the intervention
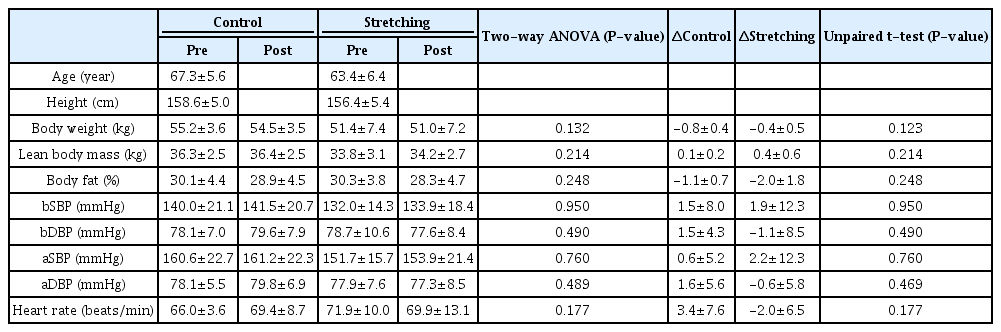
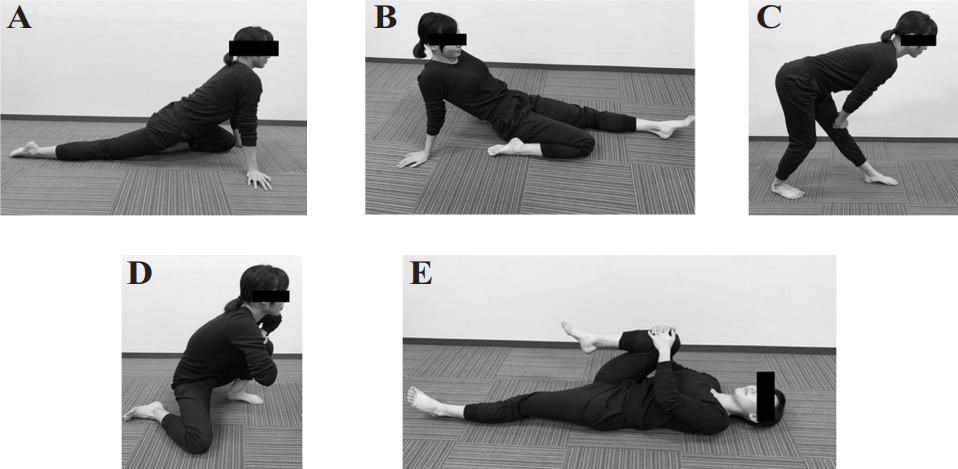
No significant differences were observed between the groups in terms of baPWV (control: pre, 1655.7 ± 296.8 cm/ s, post, 1646.4 ± 232.1 cm/s; stretching: pre, 1637.6 ± 259.9 cm/s, post, 1560.8 ± 254.7 cm/s), cfPWV (control: pre, 1253.6 ± 346.4 cm/s, post, 1223.6 ± 263.4 cm/s; stretching: pre, 1125.4 ± 204.7 cm/s, post, 1024.9 ± 164.5 cm/s), and faPWV (control: pre, 1122.7 ± 107.7 cm/s, post, 1139.9 ± 77.5 cm/s; stretching: pre, 1222.4 ± 167.5 cm/s, post, 1122.0 ± 141.1 cm/s) at baseline (Figure 2). The baPWV and cf-PWV after intervention did not differ between the groups (Figure 2). Changes in baPWV (control, -9.3 ± 91.9 cm/s; stretching, -76.9 ± 89.9 cm/s) and cfPWV (control, -30.0 ± 222.2 cm/s; stretching, -100.6 ± 117.3 cm/s) after the intervention compared to before did not differ between the control and intervention groups (Figure 3). Notably, the ANOVA revealed a significant time × intervention interaction for faPWV (F = 4.996, P = 0.0452). After the intervention, faPWV significantly decreased in the intervention group relative to that in the control group (P = 0.0031; Figure 2). Additionally, the change in faPWV (control, 17.1 ± 127.6 cm/s; stretching, -100.4 ± 55.7 cm/s) in the intervention group was significantly lower than that in the control group (P = 0.0452; Figure 3).
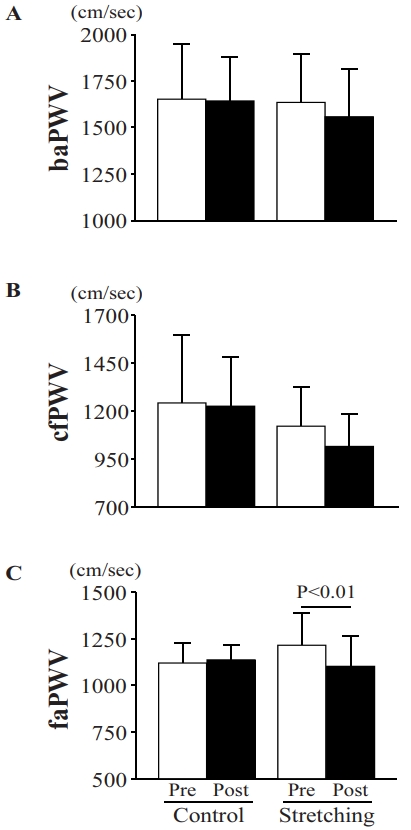
Arterial stiffness indices in baPWV(A), cfPWV(B), and faPWV(C) before and after an intervention for control vs stretching groups.
Pre, before stretching intervention; Post, after stretching intervention; baPWV, brachial-ankle pulse wave velocity; cfPWV, carotid-femoral pulse wave velocity; faPWV, femoral-ankle pulse wave velocity. Data is presented as mean ± SD.
DISCUSSION
Previous studies have shown that the systemic stretching reduces systemic arterial stiffness [11-13]. However, it is unclear whether stretching has a local and/or systemic effect on arterial stiffness. The main findings of the present study are that 4 weeks of lower-limb stretching intervention significantly decreased lower-limb arterial stiffness, but not systemic and central arterial stiffness, in the middle-aged and older women. These findings suggest that relatively short-term stretching in middle-aged and older women may be effective at the stretching sites. Therefore, it may be necessary to propose a program that considers the local effects of stretching to reduce arterial stiffness.
The present study showed a reduction in lower-limb arterial stiffness after 4 weeks of lower-limb stretching. In a previous study, a 6-month stretching intervention in the whole body significantly improved vascular endothelial function and reduced systemic arterial stiffness [12]. Furthermore, Hotta et al. demonstrated that weeks of one-legged stretching significantly enhanced endothelium-dependent vasodilation of the soleus muscle artery and increased arterial endothelial nitric oxide synthase protein levels in older rats [19]. Improvements in vascular endothelial function promote vasodilatory capacity and decrease arterial stiffness [20-22]. In a recent study, 12 weeks of stretching of the lower limb promoted vascular endothelial function in the popliteal artery [16]. Therefore, the enhancement of vascular endothelial function may be involved in the reduction of arterial stiffness via stretching interventions.
In the present study, lower-limb stretching intervention decreased peripheral arterial stiffness, but not systemic and central arterial stiffness. To reduce the degree of reduction in arterial stiffness, continuous high shear stress (e.g., shear rate) on endothelial cells induces vasodilation with increased secretion of endothelial-derived relaxation factor [20-24]. We, as well as others, have demonstrated that acute passive static stretching of the quadriceps or calf muscle increased blood flow and shear rate in the stretched leg [15,16,25-27]. Conversely, the blood flow and shear rate in the non-stretched leg did not change [15,25]. Therefore, these findings suggest that habitual repetition of the increase in shear rate induced by stretching of the lower limb may result in reduced arterial stiffness.
Previous studies have mostly used whole-body stretching to examine the effect of stretching on arterial stiffness, and few local stretches have been selected. The present study showed a reduction in arterial stiffness in the lower limbs induced by 4 weeks of lower-limb stretching, but it did not change central arterial stiffness. In a previous study, a reduction in cfPWV, an index of central arterial stiffness, was observed after a 6-week lower-limb stretching intervention, and carotid-radial PWV, an index of upper limb arterial stiffness, was reduced after 12 weeks [16]. Therefore, in this study, arterial stiffness was reduced only in the stretched site; however, in a previous study [16], a reduction in arteriosclerosis was observed in areas other than the stretched site. As the causal factor of the discrepancy between these effects of stretching, we consider that the stretching intervention period limited the effect in this study, as it was shorter than that in the previous study. Thus, if the stretch used in this study is performed for a longer period, effects can be observed at sites other than the stretched site. In conclusion, the present results indicate that a 4-week lower-limb stretching intervention significantly decreased lower-limb arterial stiffness but not systemic or central arterial stiffness. These findings suggest that lower-limb stretching may decrease arterial stiffness at the stretched site.
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (KAKENHI: 19K22828 for M. Iemitsu, #20K23302 for S. Fujie).