Effects of resistance training and protein supplementation interventions on muscle volume and muscle function: sex differences in humans
Article information
Abstract
[Purpose]
This review aimed to identify differences in the effects of co-intervention with resistance training (RT) and protein supplementation according to sex and provide meaningful information for future research on the development of exercise programs to improve muscle volume and muscle function.
[Methods]
PubMed, Science Direct, and Google Scholar were searched to identify clinical and nonclinical studies that assessed the effects of RT in older adults with sarcopenia; these studies were published between 1990 and 2023. Cross-sectional and double-blind studies (randomized controlled trials, RCTs) were examined in this review.
[Results]
The effects of parallel intervention with RT and protein supplementation on muscle volume and physical function were found to differ according to sex. Both males and females had improvements in muscle strength, muscle mass, and physical function after RT and protein supplementation; however, many studies found a greater increase in muscle volume and function in males than in females. Such difference may be due to differences in physiological characteristics between males and females.
[Conclusion]
Based on the findings of this review, the effects of combined intervention with RT and protein supplementation on muscle strength, muscle mass, and physical function to differ according to sex. Owing to these sex differences in the response and physiological characteristics caused by the parallel intervention of RT and protein supplementation, such differences must be considered to maximize the effects of RT and protein supplementation.
INTRODUCTION
Aging and lack of physical activity among elderly individuals are major public health problems [1]. Biological aging is associated with a gradual decline in muscle strength and skeletal muscle mass, and these changes have a negative correlation with human health, quality of life, and survival [2,3]. Age-related changes in the skeletal muscles are associated with a decrease in the ability to perform physical activities related to daily life and an increased risk of falls and fractures, which may ultimately increase the risk of death [4]. Only 27–44% of senior citizens in the United States meet the World Health Organization’s (WHO’s) general recommendations for adult physical activity. Those who fail to comply with these recommendations are twice as likely than those who follow them to have limited physical function [1,5]. Based on current estimates, by 2050, 200 million people worldwide will be affected by sarcopenia [6]. Exercise and protein intake are major factors that control muscle mass and they have a profound effect on muscle synthesis. Several lifestyle factors, including protein intake and reduced physical activity, contribute to a loss of muscle mass [7].
Long-term systematic resistance training (RT) increases muscle strength and mass in males and females of various ages. Muscle adaptation to RT is important for managing muscle weakness, such as sarcopenia, aging, and rehabilitation [8]. In addition, RT was found to be superior to other exercise methods at improving upper limb muscle strength, grip strength, depressive symptoms, physical performance, and walking speed [9-11]. However, the increase in muscle mass and strength induced by RT varies widely and is heterogeneous, with dependence on individual characteristics [12]. Understanding muscle adaptation to RT according to individual types allows the application of personalized exercise programs to optimally improve or maintain healthy muscle function [8].
Protein intake is important for maximizing muscle function during resistance exercises. Accordingly, protein supplementation is important for the treatment and prevention of sarcopenia [13], and can have a positive effect on the supply of essential intracellular amino acid, prevention of protein oxidation, recovery from muscle damage during exercise, and increases in muscle strength and mass [14]. Whey protein maximizes the effects of exercise and can have a positive effect on skeletal muscle anabolism [15]. Recently, athletes and the general population have started engaging in RT in parallel with protein supplementation to increase muscle strength and mass. However, to maximize the effectiveness of dietary protein supplements, proteins must be consumed according to individual characteristics [16]. Therefore, several studies have been conducted to determine the effect of protein supplementation on muscle adaptation to RT; however, studies on the differences in effectiveness of such supplementation according to sex are insufficient [17-19].
In general, females’ have less absolute muscle mass and muscle strength than males, but the decreases in muscle mass and muscle strength due to aging in females are approximately half those of males [20]. A decrease in muscle mass depends on proteolytic hormone production, the inflammatory response, fatigue, recovery time after exercise, and sex differences in muscle fiber size and type. In addition, the responses of muscle protein metabolism to exercise and nutrition differ between males and females [21]. Therefore, sex difference in muscle mass and strength according to age is one of the main factors that should be considered when developing exercise routines to minimize muscle loss [22-24]. Recently, protein metabolism has gained remarkable attention, and various measurement methods have been developed. A study examining sex differences in adaptation to resistance exercise and amino acid intake revealed that the synthesis rate of muscle proteins in males was higher than that in females [21]. Despite these developments, much is yet to be proven regarding sex differences in protein metabolism. However, considering the differences in muscle tissue and physiology between males and females, differences in protein metabolism by sex cannot be ruled out.
Many previous studies have examined the changes in muscle mass and muscle function following RT or protein supplementation; however, the effectiveness of the combined intervention has not been evaluated in many studies. In addition, despite evidence that the effects of these combined interventions can vary depending on personal characteristics, such as sex, age, and race, few studies have performed comparisons according to these attributes, especially according to sex. Therefore, the aim of this review was to identify the differences in the effects of RT and protein dietary supplementation on muscle strength, muscle mass, and muscle function according to sex, and provide meaningful information for future research on individuals with sarcopenia or the development of exercise programs to improve and prevent muscle mass and muscle function reduction.
METHODS
PubMed, Science Direct, and Google Scholar were searched to identify clinical and nonclinical studies that determined the effects of RT in older adults with sarcopenia; these studies were published between 1990 and 2023. The keywords for the search were “sex differences and muscle,” “sex differences and RT effects,” “sex differences and protein metabolism,” “sex differences and sarcopenia,” and “RT and Protein supplementation.”
This review comprised cross-sectional and double-blind studies (randomized controlled trials, RCTs). Animal and experimental studies were excluded from this review. In addition, studies with small sample sizes, those that did not adequately specify the selection criteria, or included groups of patients receiving medication for another disease that affected bone or muscle metabolism were excluded.
RESULTS AND DISCUSSION
Effects of RT and Protein Supplementation on Muscle Strength: Sex Differences
Several studies found a strong correlation between muscle strength and sarcopenia. In patients with sarcopenia, muscle mass decreases at a rate of approximately 1% per year while muscle strength decreases at a relatively faster rate [25]. Decreased muscle strength is a strong predictor of physical injury and diseases in humans [26]. RT has a positive impact on improving muscle function, muscle mass, and bone health, and preventing musculoskeletal and metabolic diseases to maintain and improve an individual’s quality of life. RT is a type of exercise that provides various health benefits, especially in elderly people [27,28]. Therefore, maintaining and increasing muscle strength via RT are important for improving muscle function and preventing physical injury and disease [29,30].
A nutritional Intervention is an important strategy for improving and maintaining muscle strength, muscle mass, and muscle function [31,32]. According to a previous study, the combination of RT and protein supplementation increased muscle protein synthesis to a greater extent than RT [33]. In older adults, protein supplementation has been demonstrated to increase overall lean body mass and grip strength only when combined with RT [34]. The combination of RT and protein supplementation has been shown to induce increased plasma amino acid levels and stimulate muscle protein synthesis [35]. This combination has also been proven to increase individual muscle strength and lean body mass; however, studies considering personal characteristics, such as sex, age, and race, are limited.
Sex-specific physiological characteristics must be considered when RT and protein supplementation are recommended to improve muscle strength and prevent sarcopenia. Body composition, including muscle mass and body fat, generally varies according to sex. Therefore, the effects of RT and protein supplementation on human muscle strength must be considered according to sex [36]. In general, males have higher muscle masses and lower body fat percentages than females. Moreover, there are differences in the type of muscle and the distribution of muscles in the musculoskeletal system according to sex [37]. Females have relatively less muscle strength as they have a higher distribution of type 1 muscle fibers than males [38]. Despite the differences in physiological characteristics according to sex, studies on sex differences in the effects of RT and protein supplementation on muscle strength are lacking.
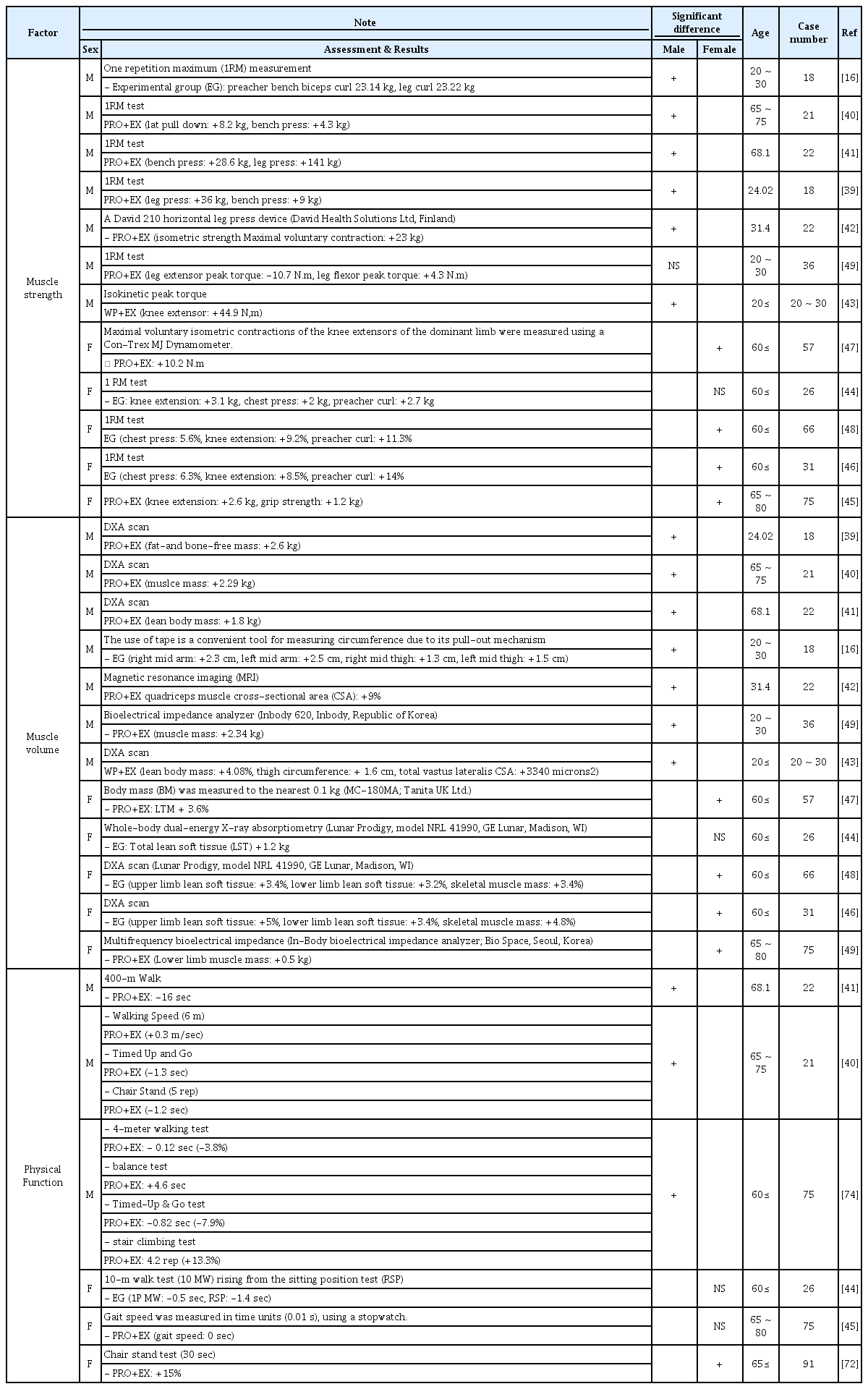
In 2019, a study comprising 18 males aged 20–30 years examined the changes in muscle strength in response to co-treatment with RT and protein supplementation. After 12 weeks of intervention, male muscle strength improved significantly (preacher bench biceps curl: +23.14 kg, leg curl: +23.22 kg) [16]. In 2018, another study comprising young males examined the effects of RT and mixed protein intake on muscle strength and found significant improvements in muscle strength (leg press: +36 kg, bench press: +9 kg) [39]. In 2015, 21 males aged 65–75 years were monitored to derive their response to the combined intervention of RT and protein supplementation. In this study, the muscle strength of males significantly improved (lat pulldown: +8.2 kg, bench press: +4.3 kg) [40]. In 2014, another study comprising elderly males examined the effects of RT and whey protein supplementation on muscle strength and found significant muscle strength improvement owing to the treatment (bench press: +28.6 kg, leg press: +141 kg) [41]. Many studies that investigated the effects of RT and protein supplementation on muscle strength in males reported similar results (Table 1, Table 2) [42,43]. In 2019, we examined the effects of RT and protein supplementation on muscle strength in 26 females older than 60 and found some improvements in muscle strength; however, the result was not significant (knee extension: +3.1 kg, chest press: +2 kg, preacher curl: +2.7 kg) [44]. In 2018, another study examined the effects of RT and whey protein supplementation on muscle strength in 75 elderly females and found significant improvements in muscle strength (knee extension: +2.6 kg, rip strength: +1.2 kg) [45]. In 2017, a study comprising 31 females aged 60 or older found improvements in muscle strength after concurrent interventions of RT and protein supplementation (knee extension: +8.5%, chest press: +6.3%, preacher curl: +14%) [46]. Of note, many studies that investigated the effects of RT and protein supplementation on muscle strength in females have reported similar results (Table 1, Table 2) [47,48]. Comparing the results of studies with males and females confirmed a greater improvement in muscle strength in males than females after RT and protein supplementation. In addition, among the studies on females, no significant improvement was found. Nevertheless, some studies with males did not report a significant increase in muscle strength [49]. By comparing these findings, a sex difference can be observed in the muscle strength response to RT and protein supplementation. Nevertheless, proving this sex difference is insufficient as studies have not directly compared the effects of combined interventions of RT and protein supplementation by sex (Table 1, Table 2).
Sex differences in the effects of resistance exercise and protein supplementation in humans on muscle strength, muscle mass, and muscle function.
Effects of RT and Protein Supplementation on Muscle Volume: Sex Differences
Decreased muscle mass causes insulin resistance, a reduced ability to control blood sugar, and an increased prevalence of cardiovascular and metabolic diseases. This decrease is also directly related to mortality because it affects the occurrence of cardiovascular diseases and cancer [50,51]. Muscle mass is a major evaluation factor for sarcopenia caused by aging, and maintaining or improving individual muscle mass is an important factor in healthy aging. RT has been demonstrated to have a remarkable effect on the maintenance and improvement of muscle mass [52]. Although the effect of RT on the body muscle mass varies according to personal characteristics, such as sex, age, and race, few studies have been conducted on RT prescriptions that consider these individual differences.
Protein supplementation stimulates anabolic hormone components, such as the growth hormone, insulin-like growth factor (IGF-1), and testosterone, to induce protein synthesis, resulting in increased muscle mass [53]. Protein supplementation before and after resistance exercise also increases muscle protein levels during recovery and protein synthesis after exercise [54]. Protein supplementation can further enhance the effects of increased muscle mass on RT in elderly individuals [55,56]. However, studies investigating the combined effects of RT and protein supplementation on muscle mass have yielded conflicting results, thereby leading to a lack of proof of the efficacy of protein supplementation. Several studies have investigated the effects of RT and protein supplementation in older adults; however, only few studies have directly compared the differences according to individual characteristics [56-58].
In 2018, Mathias et al. found that eight weeks of RT and protein supplementation significantly improved muscle mass in males (fat-and-bone-free mass: +2.6 kg) [39]. Another 2018 study comprising 22 young males found an increase in muscle mass after co-therapy (quadricep muscle cross-sectional area, +9%) [5]. According to a 2015 study by Mathieu et al., 12 weeks of RT and protein supplementation significantly increased muscle mass in elderly males (muscle mass: +2.29 kg) 40 . In 2014, 22 elderly males had significant increases in muscle mass owing to a combination of 12-week RT and protein supplementation (lean body mass, +1.8 kg) [41]. Several studies investigating the effects of RT and protein supplementation on muscle strength in males reported similar results [16,49]. According to Hellen et al., 12 weeks of RT and protein intervention in elderly females resulted in a slight improvement in muscle mass; however, this result was not significant (total lean soft tissue: +1.2 kg) [44]. In 2018, a study surveyed 75 elderly females to determine the muscle mass following combined intervention with RT and protein for 24 weeks. Based on the results, a significant improvement in muscle mass (lower limb muscle mass: +0.5 kg) was found [45]. Similar results have been reported in several studies examining the effects of RT and protein supplementation on muscle mass in females [44,47,48]. By comparing the results between males and females, muscle mass increase in males was confirmed to be greater after intervention with RT and protein supplementation than in females. In addition, among studies comprising females, we confirmed that no significant improvement was found. Therefore, a sex difference exists in the muscle mass response to RT and protein supplementation. Nevertheless, further studies are needed to demonstrate these sex differences, as no studies have evaluated the effects of combined RT and protein supplementation interventions on muscle hypertrophy responses by sex (Table 1, Table 2).
Effects of RT and Protein Supplementation on Muscle Function: Sex Differences
The main issue associated with decreased muscle strength and mass is the decrease in physical ability due to muscle dysfunction. This reduction increases the possibility of falls and various musculoskeletal diseases, and degrades the quality of life of individuals [59,60]. Physical function, muscle strength, and muscle mass are key factors in the diagnosis of sarcopenia [61]. RT has been proven to improve physical functions, such as individual walking speed, stride, flexibility, and balance ability [62-64]. Several previous studies have investigated the effects of RT interventions on physical function in males and females; however, only few studies have directly compared the effects of RT based on sex [65,66].
Among the many factors that lead to decreased muscle mass, inadequate protein intake and decreased physical activity are most important [67,68]. Several strategies are used to prevent muscle loss, including protein supplementation, RT, and hormone replacement therapy [69]. Increasing muscle mass and physical function are major factors in preventing and improving sarcopenia; thus, these need to be treated as important factors [70]. Considering the importance of muscle strength and body function from this point of view, analyzing the effectiveness of RT and protein supplementation in improving sarcopenia symptoms is important [71]. Several studies have identified significant incremental effects of RT and protein supplementation on body functions. However, only few studies have directly compared the effectiveness based on personal characteristics [40,72,73]. As the effects of RT and protein combination may vary depending on physiological characteristics, such as sex, age, and race, various approaches are needed to maximize the effects of RT and protein supplementation considering personal characteristics.
A study published in 2017 found that 12 weeks of RT and protein supplementation intervention improved physical function in elderly males (walking speed: +0.3 m/s, timed up and go: -1.3 s, chair stand: -1.2 s) [40]. In 2021, a study involving 75 males aged 60 or older investigated the effects of 12-week RT and protein supplementation and found significant improvements in physical function (4 m walking test: -1.2 s, timed up and go: -0.82 s, balance test: +4.6 s, stair climbing test: +4.2 rep) [74]. A 2014 study by Matthew et al., which comprised 22 elderly men, found that RT and protein supplementation had a positive effect on improving body function (400 m walk: -16 s) [41]. Conversely, we examined the effects of RT and protein supplementation in 26 females aged 60 or older in 2019 and found some improvement in physical function; however, the result was not significant (10 m walk test: -0.5 s, rising from the sitting position test: -1.4 s) [44]. A 2016 study involving 75 females aged 65 to 80 years investigated the response of physical function to concurrent interventions of RT and protein supplementation and found a slight improvement in physical function; however, this improvement was not significant (gait speed: + 0 s) [45]. Altogether, the results of these studies highlight sex-specific differences in the response of body function to combined interventions of RT and protein supplementation. Studies comprising males have revealed improvements in physical function while studies comprising females have not reported significant increases in physical function. Nonetheless, studies comprising females have found increases in physical function but generalizing the results is difficult to perform as these studies did not directly assess sex differences [75]. Therefore, further studies are needed to demonstrate the sex differences in muscle strength, muscle mass, and body function owing to co-treatment with RT and protein supplementation (Table 1, Table 2).
Sex Differences in Adaptation to RT and Protein Supplementation Based on Physiological Characteristics
The differences in the effects of combined RT and protein supplementation on individuals according to sex remain unclear. However, this potential sex difference may be due to differences in physiological characteristics, such as the the type of muscle, protein metabolism, and hormones [75,76]. An investigation of the structural properties of the skeletal muscle revealed that sex differences may affect responses to RT and protein supplementation. Inborn sex differences cause significant differences in the morphological composition of skeletal muscles. Males have more skeletal muscles than females, which contributes to greater maximum muscle strength in males [76,77]. Females have relatively less muscle mass and muscle strength than males but have more type I muscle fibers that are resistant to fatigue. In contrast, males have relatively more muscle mass and muscle strength, and more type II muscle fibers that are vulnerable to fatigue. These sex-dependent differences in muscle fiber structure can also affect the responses to RT and protein supplementation. In a study involving RT for nine weeks, increased muscle mass was observed in both type I and type II muscle fibers in elderly males [78]. In contrast, a 12-week intervention of RT in older females led to a significant increase in type II muscle fibers but not type I muscle fibers [79]. Differences in muscle type and muscle distribution in the musculoskeletal system may cause sex differences in the effects of combined interventions of RT and protein supplementation [80].
Another physiological trait that can affect the response to combined RT and protein supplementation is the levels of hormones, which affect protein synthesis. Several circulatory expression factors (IGF-1 and testosterone) are presumed to be important for muscle protein balance based on muscle protein synthesis [81]. IGF-1 is upregulated in overloaded muscles and is associated with an increase in myogenic regulators, such as myogenin [82]. When stimulated by a load or myotoxicity, the expected myogenic expression in older muscles is reduced or delayed compared to that in younger muscles [83,84]. Based on this information, sex differences in individual muscle growth due to concurrent interventions of RT and protein supplementation may be associated with muscle IGF-1 levels. Owing to a lack of clear evidence of differences in the response of muscle protein metabolism to RT and protein supplementation, the higher metabolism of protein in males than females may be attributed to hormonal differences. Testosterone treatment in males increased muscle protein synthesis and IGF-1 expression, suggesting that testosterone and IGF-1 are factors regulating muscle metabolism, and are positively correlated with each other. In general, testosterone levels are 10-fold higher in males than in females. Testosterone is an important anabolic hormone, and differences in testosterone concentration can lead to sex differences in protein synthesis during exercise and protein supplementation [85].
Muscle loss is a decreased response of muscle protein synthesis to generally strong anabolic stimuli, such as protein intake and RT [86,87]. A recent study highlighted significant age- and sex-related differences in the regulation of muscle protein synthesis [88]. These data suggest that muscle protein synthesis in males may exhibit a different response to exercise and nutritional supplementation than that in females; however, expanding our conclusions is difficult. As protein degradation and synthetic responses to combined interventions of RT and protein supplementation by sex have not been reported, the problem must be addressed according to sex differences.
According to various studies, rapamycin complex 1 (mTORC1) plays a decisive role in skeletal muscle hypertrophy as it is activated after resistance exercise in humans [89,90]. The ubiquitin-proteasome system is a major proteolytic system. Previous studies reported that RT significantly increased the mRNA expression of muscle RING-FINGER protein-1, which promotes protein ubiquitination [91]. Essential amino acids are known to induce anabolic reactions in muscles, including mTORC1 activation [33,92]. In addition, branched-chain amino acids (BCAAs) have been found to more effectively stimulate mTORC1 when combined with essential amino acids [93]. RT and protein supplementation were previously found to effectively increase the activation of mTORC1 signaling, suggesting that protein supplementation is useful for increasing the effects of RT in males [94].
CONCLUSION
Muscle function decreases with age, resulting in sarcopenia, which is directly related to health and the quality of life. RT has a remarkable effect on the prevention of sarcopenia and improvement of muscle strength and mass. Protein supplementation maximizes the effects of RT and improves muscle mass and hypertrophy. This review suggests the existence of sex differences in the effects of co-treatment with RT and protein supplementation on muscle strength, muscle mass, and physical function. Upon examining the studies covered in this review, the synergistic effects of RT and protein supplementation were identified to be greater in males than in females. In most studies, muscle strength, muscle mass, and physical function in males increased after combined intervention, wih a greater increase found in males than females. In studies comprising females, the muscle strength, muscle mass, and physical function in some females increased; however, this increase was lower than that in males; a study also found no significant increase in these indices. As sex differences exist in the response and physiological characteristics caused by the combined intervention of RT and protein supplementation, these sex differences must be considered to maximize the effects of RT and protein supplementation. However, only few studies directly compared sex. Further, understanding the combined effects of RT and protein supplementation is limited as different results are obtained according to individual characteristics. Therefore, future studies must comprehensively investigate and compare sex differences in the effects of RT and protein supplementation to present effective strategies to improve muscle mass and function.

 PRO+EX: +10.2 N.m
PRO+EX: +10.2 N.m