The role of continuous glucose monitoring in physical activity and nutrition management: perspectives on present and possible uses
Article information
Abstract
[Purpose]
Continuous glucose monitoring (CGM) is on the rise as the prevalence of obesity and diabetes increases. This review aimed to explore the use of CGM and its potential novel applications in physical activity and nutrition management.
[Methods]
We searched PubMed, Web of Science, and Wiley Online Library databases using the keywords ‘continuous glucose monitor,’ ‘nutrition,’ ‘physical activity,’ and ‘numerical modeling.’
[Results]
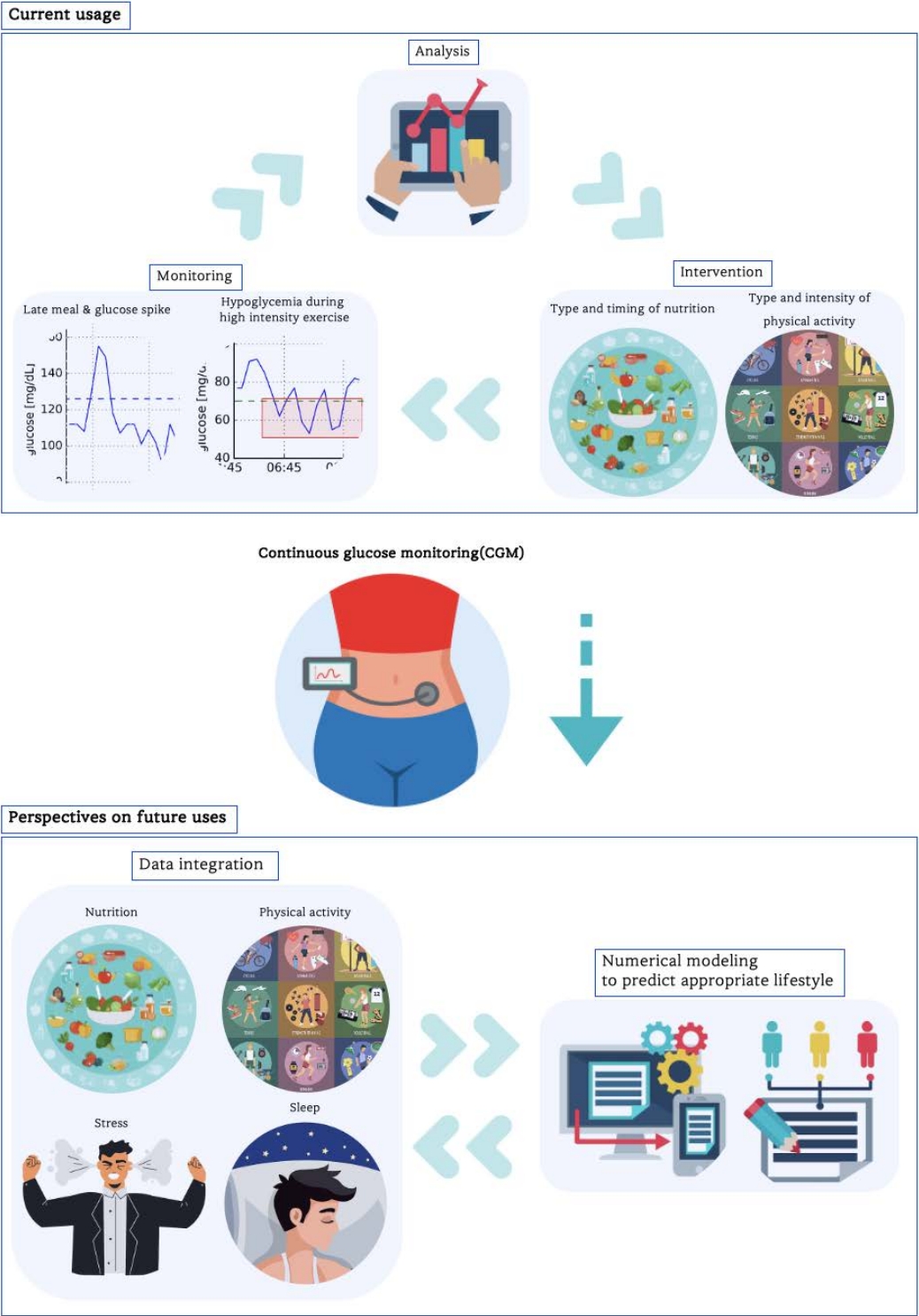
Continuous blood glucose measurement is useful for individuals with obesity and diabetes. Long-term blood glucose data allow for personalized planning of nutritional composition, meal timing, and physical activity type and intensity, as well as help prevent hypoglycemia and hyperglycemia. Thus, understanding the limitations of CGM is important for its effective use.
[Conclusion]
CGM systems are being increasingly used to monitor and identify appropriate blood glucose controlling interventions. Blood glucose level is influenced by various factors such as nutrient composition, meal timing, physical activity, circadian rhythm, and cortisol levels. Numerical modeling can be used to analyze the complex relationship between stress, sleep, nutrition, and physical activity, which affect blood glucose levels. In future, blood glucose, sleep, and stress data will be integrated to predict appropriate lifestyle levels for blood glucose management. This integrated approach improves glucose control and overall wellbeing, potentially reducing societal costs.
INTRODUCTION
According to the energy balance concept, obesity is the accumulation of excess body fat as a result of an imbalance between calorie intake and calorie expenditure [1]. Obesity is an important public health issue because it can cause various health problems such as diabetes [2], high blood pressure [3,4], heart disease [5,6], stroke [7,8], and some cancers [9-11] as well as also be associated with physical limitations [12], poor quality of life (QOL) [13], and increased mortality risk [14,15]. World Health Organization (WHO) has recognized obesity as a global health crisis that affects people of all ages, sexes, and socioeconomic statuses [16]. The worldwide prevalence of obesity continues to rise and more than half of the global population is expected to become obese by 2030 [17].
Obesity rate has increased in South Korea as well, owing to changes in lifestyle and dietary habits. According to the 2018 Korea National Health and Nutrition Examination Survey, obesity and diabetes rates in South Korea were 35.7% and 14.4%, respectively [18]. In 2020, approximately 40% adults were classified as overweight or obese [19]. Current lifestyle, characterized by decreased physical activity, increased evening activity, and poor dietary habits, causes serious problems by promoting energy imbalance, which increases obesity and diabetes risk [20].
Insulin, a hormone produced by beta cells in the pancreas, plays a pivotal role in regulating blood glucose levels [21]. Insulin regulates blood sugar levels by stimulating glucose uptake from the bloodstream into cells, where it is utilized as energy or stored as glycogen. Insulin also prevents the liver from releasing excess glucose into blood [22]. The normal fasting blood glucose level range (measured after at least 8 h without food) is typically 70–100 mg/dL or 3.9–5.6 mmol/L [23]. Blood glucose levels measured 2 h after eating (postprandial) should not exceed 140 mg/dL (7.8 mmol/L). Individuals with obesity often experience type 1 or 2 diabetes, which are characterized by insufficient insulin production or decreased insulin sensitivity, respectively [24]. Thus, glucose control and improved insulin sensitivity are crucial in individuals with obesity, diabetes, and metabolic disorders [25,26]. Consistent high (hyperglycemia) or low (hypo-glycemia) sustained blood glucose levels have serious health consequences. Hyperglycemia causes diabetes, cardiovascular disease, and kidney disease [27,28]. Moreover, severe hypoglycemia causes consciousness loss, seizures, and even death if not treated immediately [29,30]. Frequent hypoglycemia also reduces an individual’s quality of life, as it can cause anxiety, fear of hypoglycemia, and avoidance of physical activity or social circumstances [31]. Both hyperglycemia and hypoglycemia causes cognitive impairment, which can affect concentration, memory, and decision making [32,33], as well as contribute to mood disorders, such as mood swings, irritability, and depression [34]. As the prevalence of obesity-related diseases increases, it becomes more important to find effective ways to manage blood glucose levels before they become diseased.
Continuous glucose monitoring (CGM) has resulted in a new era of blood glucose measurements and provides real-time insights into blood glucose levels [35]. It involves using small sensors under the skin to continuously measure glucose levels in the interstitial fluid surrounding cells [36,37]. The measurements are then transmitted to a receiver or smartphone application, which allows the individual to monitor glucose levels in real time. CGM has become increasingly important in blood glucose management as the prevalence of diabetes increases [38,39]. The technical and clinical use of CGM has been widely reviewed [40-45]. However, no reviews have focused on how their use in physical activity and nutrition management and their possible future applications. This review aimed to analyze how CGM has been used in physical activity and nutrition management and suggest possible future applications based on numerical modeling.
EXPLORING THE ROLE OF CGM IN PHYSICAL ACTIVITY AND NUTRITION MANAGEMENT
Unveiling the Vital Role: Importance of CGM Data
CGM data play an important role in diabetes management by providing valuable insights into blood glucose levels [46]. This continuous real-time information, collected every 1–15 min, is critical for individuals with diabetes [47].
CGM allows an understanding of blood glucose trends over long time periods such as hours, days, and even weeks [48,49]. CGM data can be used to understand how different factors, such as nutritional choices, physical activity, stress, and medication use, affect blood glucose levels [50-52]. Alerts to low or high blood glucose levels allow individuals to take immediate action to prevent rapid fluctuations in blood sugar [53]. It also evaluates the ratio of time spent within a pre-defined target blood glucose range, giving an excellent picture of blood glucose level maintenance within the recommended range (time-in-range; TIR) [54-57]. These findings provide a foundation for personalized therapeutic interventions. The impact of different foods on blood glucose levels can be observed using CGM [58]. By measuring the postprandial blood glucose changes in detail, individuals can make data-driven decisions regarding meal composition and timing, leading to balanced and manageable blood glucose control.
Physical activity should be performed with care, particularly for people with diabetes because of the response of blood glucose levels to physical activity [59]. The effect of physical activity on blood glucose levels can be tracked using CGM. With CGM data, exercise routines and insulin doses can be fine-tuned to reduce exercise-induced blood glucose fluctuations and achieve consistent glucose management. CGM can help identify overnight blood glucose trends and help users determine the need for insulin adjustments [60]. This proactive approach can minimize the risk of nighttime hypoglycemia or hyperglycemia, thereby ensuring restful and safe sleep. CGM data can also be used to promote discussions with medical professionals at the point-of-care about appropriate treatment strategies and adjustments [61]. This will help to effectively improve overall diabetes management and personalize treatment plans to meet individual needs.
CGM Use for Enhancing Nutrition Insights
CGM is useful for understanding the complex relationship between nutrition, obesity, and diabetes.
This reveals how different foods and dietary patterns affect people with obesity. It is not the only solution for obesity management, but CGM can help analyze metabolic response to different foods and inform nutritional choices [62]. The effects on blood glucose differ for each individual and are influenced by several factors, such as genetics, metabolism, metagenomics, and diet. Therefore, personalized guidance may be more appropriate than one-size-fits-all impact guidelines [63]. A study comparing the effects of brown rice (BR) and white rice (WR) on 24 h glycemic and insulinemic responses of overweight Asian Indians found that BR consumption reduces the risk of diabetes and related complications by reducing 24 h glucose and fasting insulin responses [64]. This study suggests that BR is a healthier alternative to WR. CGM data can show how a particular meal affects blood glucose levels over time, allowing the design of a diet to promote blood glucose stability and prevent rapid blood glucose changes. Carbohydrate intake should be adjusted to manage blood glucose fluctuations owing to the complex relationship between the carbohydrates in different foods and blood glucose levels [64]. The pilot study explored the feasibility and safety of time-limited eating (TLE) combined with CGM in adolescents with obesity [65]. This study found high adherence to TLE and CGM with no significant differences in weight loss, energy intake, quality of life, or eating behavior [65]. This study found that intra-individual variability in nutrition-related lifestyle behaviors, such as meal timing, eating windows, food intake, movement behaviors, sleep conditions, and body weight, was significantly correlated with mean blood glucose levels66. Long sedentary period and total sleep duration are associated with glucose variability. Earlier dinners and shorter eating windows improve glucose control [66]. An effective weight management strategy can be developed by optimizing nutrient intake and meal timings based on glucose response.
CGM is also useful in assessing the relationship between glucose control and nutrition in individuals with diabetes [67]. This can help fine-tune insulin doses to match carbohydrate intake [68-70]. CGM data can help guide the timing of mealtime insulin doses, helping timely insulin administration to prevent postprandial blood glucose spikes [71-73]. This study investigated the effects of carbohydrate distribution on postprandial glucose peaks in individuals with type 2 diabetes [74]. Twenty-three participants were randomly assigned to four interventions, each with varying carbohydrate content. The results showed that even carbohydrate distribution did not enhance blood glucose control, although a carbohydrate lunch offered an optimal postprandial profile. This study examined for how high glycemic index (HGI) and low glycemic index (LGI) meals affected blood glucose levels in youths with type 1 diabetes [75]. The results showed low daytime mean blood glucose, blood glucose area (>180 mg/dL), and high blood glucose index when consuming LGI meals, but no differences in daytime blood glucose area (<70 mg/dL) between HGI and LGI [75]. Similar to obesity, people with diabetes can use CGM data to analyze the impact of meals on blood glucose levels, which can be used to guide nutritional composition.
CGM Use for Optimizing Physical Activity
CGM helps us understand the impact of physical activity on obesity and diabetes management.
The CGM provides data on how physical activity type and intensity affect blood glucose levels in people with obesity. This study compared the effects of high-intensity interval training (HIIT) and continuous moderate-intensity exercise on postprandial hyperglycemia in overweight or obese adults [76]. The results showed that HIIT significantly reduced the incremental area under the curve after dinner and postprandial glucose spikes after breakfast, suggesting that HIIT has a prolonged effect on postprandial hyperglycemia. CGM data can reveal the relationship between different forms of physical activity and glucose levels [76-78]. Thus, one can choose a physical activity that helps maintain stable glucose levels and avoid rapid glucose fluctuations, particularly in people with insulin resistance due to obesity [79]. This study investigated hypoglycemia during moderate-intensity exercise in non-obese and obese individuals with and without type 2 diabetes [80]. The results showed elevated glucose responses and high decrease in glucose concentration during the evening exercise. Obese males had high insulin drops. Moderate-intensity exercise decrease glucose concentrations; however, many remain asymptomatic [80]. Proper physical activity and meal timing around physical activity can also be planned based on CGM data. Pre-workout meals or snacks should be adjusted based on blood glucose levels to optimize energy levels and avoid glucose imbalance during physical activity. The insights gained from CGM can help plan physical activities to improve overall well-being.
CGM is also useful in people with diabetes. This study examined the effects of structured exercise on glucose levels in adults with type 2 diabetes [81]. The results showed that both acute and chronic exercise can improve 24 h glucose profiles, and the timing of exercise and participant sex influence the heterogeneity of acute glycemic improvements [81]. This can help make informed decisions about physical activity and timing based on CGM data. This systematic review examined the effects of exercise on glycemic control in type 2 diabetes [82]. According to a meta-analysis of 11 studies focusing on postprandial glucose, exercise significantly decreased average glucose concentrations and time spent in hyperglycemia, but did not affect the daily time spent in hypoglycemia or fasting glucose [82]. Physical exercise is crucial for managing type 1 diabetes; however, acute exercise increases the risk of dysglycemia [83]. The fear of hypoglycemia is a barrier to exercise. CGM and intermittently scanned CGM (isCGM) systems can help manage glycemic during exercise [83] and determine when to adjust the insulin dose before exercise, based on the glucose levels and expected effects of exercise. Since muscles become insulin-sensitive, this period is critical for optimizing glycemic control [84-87].
Recognizing Constraints: Limitations
CGM has made great progress in diabetes management; however, its limitations are similar to those of other technologies. Thus, its limitations should be understood for effective data utilization and analysis. The accuracy of CGM has improved over time compared to that of traditional fingerstick method for blood glucose measurement, but it still exists [88-90]. As CGM sensors measure glucose levels in the interstitial fluid, they may not accurately match blood glucose levels, particularly when glucose level changes rapidly, such as after a meal or during exercise [89]. There might be a slight latency between blood glucose level changes and blood glucose readings with CGM, which affects the timing of appropriate interventions during rapid blood glucose fluctuations; therefore, it is important to be aware of this [91]. Most CGM systems should be calibrated periodically using fingerstick measurements as inaccurate or infrequent calibration can affect data reliability [92-95]. Attaching a sensor to the CGM can be inconvenient [96]. Some individuals may experience difficulty in applying the sensor or irritation at the sensor site [43,97]. Skin reactions such as rash, itching, or inflammation may occur at the sensor attachment site with prolonged CGM sensor use. CGM use requires active user participation in sensor attachment, calibration, and data analysis. There may be inaccurate results owing to user errors or inappropriate procedures. Constant data stream from CGM devices can be stressful for some people. Constant monitoring and analysis causes “data fatigue” [98,99]. Technical issues with CGM devices, such as sensor failure or synchronization, can disrupt data collection and affect usability [100,101].
PERSPECTIVE ON THE FUTURE
Many institutions use CGM systems to monitor and identify appropriate interventions and possible applications. Researchers are also working to develop a noninvasive CGM102. The CGM era is dawning and substantial changes are expected in the future. CGM should be used to monitor feedback changes in blood glucose levels over time. However, very little work has been done to analyze and apply numerical modeling to CGM-generated data. The CGM data should be analyzed to develop possible numerical modeling techniques and prediction techniques to prepare for the future.
Blood glucose is not simply the conversion of food into sugar; it is influenced by many factors, such as nutrient composition, meal timing, the presence or absence of physical activity, circadian rhythms, and cortisol levels, and therefore requires a systematic approach [103-106]. Wearable devices are ubiquitous, can track sleep patterns, and provide data on stress levels, such as heart rate variability. Stress disrupts sleep patterns, reduces sleep quality, and alters sleep duration. Sleep disruption can eventually affect glucose metabolism and regulate blood glucose levels. Numerical modeling, also known as computational or mathematical modeling, uses mathematical equations and computer simulations to represent and analyze complex real-world systems, phenomena, or processes. Numerical modeling can be used to analyze the complex relationships among stress, sleep, nutrition, and physical activity, which affect blood glucose levels. Currently, CGM data are used independently; however, in the future, blood glucose, sleep, and stress data will be integrated. Numerical models can be used to predict how stress-induced sleep disruption leads to specific blood glucose fluctuations. One study found that sleep deprivation was associated with nighttime blood glucose spikes. Machine learning algorithms can help identify specific sleep-related factors (such as bedtime routine and sleep duration) that have the greatest impact on blood glucose levels. Physical activity can reduce sleep deprivation-mediated blood glucose spikes [107]. Using the CGM, physical activity type and intensity can be determined and applied optimally. Some people experience an immediate blood glucose spike when stressed, whereas others may experience a delayed effect, indicating chronic stress. Chronic stress increases cortisol levels over time, which are likely to negatively affect cognitive function. Physical activity can reduce stress, contribute to cognitive function, and help manage blood glucose levels [108,109]. Armed with insights from CGM data, individuals can develop stress-management strategies that fit their unique patterns. For patients with diabetes, healthcare professionals can adjust medication dosages or insulin regimens based on stress-related blood glucose patterns. This integrated approach identifies the interconnectedness of stress, sleep, nutrition, and physical activity, which affect glucose control, allowing individuals to take a holistic approach to their health by improving not only glucose control, but also overall well-being. Based on these insights, appropriate lifestyle levels can be predicted for a growing number of individuals with obesity and diabetes, which could help reduce societal costs.
Acknowledgements
This research was supported by a College of Education, Korea University Grant (2023).
The authors have no financial, consulting, institutional, or other relationships that may lead to bias or conflicts of interest.