The effect of voluntary exercise on light cycle stress-induced metabolic resistance
Article information
Abstract
[Purpose]
Disruption of circadian genes affects metabolic homeostasis. Regular exercise programs prevent metabolic dysfunction and alter circadian gene expression In this study, we investigated whether exercise affects light stress-induced circadian rhythm derangement and metabolic resistance.
[Methods]
A circadian rhythm derangement mouse model was designed by extending the light exposure by two hours (14 L/10 D) for three weeks. Nine-weekold male mice were single-caged and divided into four groups: sedentary groups with or without light stress, and voluntary wheel-trained groups with or without light stress. In addition, differentiated myotubes were cultured in the presence of dexamethasone with or without 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR). The comprehensive laboratory animal monitoring system was used to analyze the metabolic changes in mice. Moreover, reverse transcription-polymerase chain reaction (RT-PCR) was used to quantify the mRNA expression levels of circadian genes in animal and cell culture models.
[Results]
Three weeks of light stress reduced the running distance and increased the weight of mice. In addition, VO2 consumption and heat production were increased during the night cycle under non-stress conditions but not under stress conditions. PCR analysis revealed that exercise and stress altered the expression levels of circadian genes in the hypothalamus and quadriceps muscles. mRNA expression levels of period circadian regulator 1 were downregulated in the quadriceps muscles of the stressed sedentary group compared to that in muscles of the non-stressed sedentary group. Furthermore, differentiated myotube cells cultured in the presence of dexamethasone, with or without AICAR, showed distinct oscillation patterns at various time points.
[Conclusion]
Our study demonstrates that exercise partially prevents metabolic disruption by regulating the circadian gene expression in skeletal muscles.
INTRODUCTION
Nutritional imbalance and metabolic malfunction are serious health issues [1-3]. The causes of these metabolic conditions include genetic and non-genetic factors, such as excessive food intake, unhealthy eating habits, socioeconomic factors, lifestyle, and lack of exercise [4,5]. Lifestyle disruption due to light at night is a risk factor for metabolic diseases5. Innovations in information technology and logistics have resulted in most developed countries operating 24 h daily [3]. Notably, 1.27 to 1.97 million people worldwide and 10–15% of all workers in South Korea are estimated to be night workers [6]. Many studies on shift workers suggest that night work affects the metabolic balance of the body and causes obesity and other metabolic disorders that threaten human health [3-5].
Human body has various cyclical rhythms to maintain homeostasis7. These repetitive biological processes are defined as biological rhythms, one category of which is the circadian rhythm [7]. Mammalian circadian rhythms are triggered by a cell-autonomous transcriptional autoregulatory feedback loop. The core clock genes include CLOCK and basic helix-loop-helix ARNT-like 1 (BMAL1), which encode activators, and period circadian regulator (PER)-1, PER2, cryptochrome (CRY)-1, and CRY2, which encode inhibitors [8]. In mice, activation of CLOCK–BMAL1 occurs primarily during the day [9]. PER and CRY accumulate in the late afternoon and evening by inducing the transcription of Per and Cry genes in the afternoon [10]. These biological rhythms are maintained by an endogenous circadian system consisting of a central clock in the suprachiasmatic nucleus (SCN) of the hypothalamus and peripheral clocks. Disruption of the circadian system causes serious problems, such as increased risk of coronary disease and metabolic syndrome, sleep deprivation, and various psychological disorders [11].
Lifestyle modifications involving increased physical activity can lead to improvements in metabolic disorders [12-14]. Participation in moderate-intensity exercise improves the glucose metabolism and insulin resistance and lowers the mortality rate due to cardiovascular disease [12,14]. Exercise also regulates circadian gene expression [15]. Skeletal muscle circadian genes are affected by a 12-week exercise intervention in prediabetic subjects [15]. Clock- and BMAL1-deficient mice exhibit reduced maximum muscle strength and mitochondrial volume in skeletal muscles [16]. However, Clock-deficient mice exhibited no effect on endurance during exercise performance [17].
Effect of exercise on the regulation of circadian genes under light-cycle stress remain unknown. Therefore, in this study, we aimed to investigate whether exercise affects metabolic resistance under light cycle stress and determine the molecular mechanism underlying exercise-activated signaling during Clock gene oscillation under stress conditions in skeletal muscle cells.
METHODS
Ethics approval
Ethical approval and consent to participate in this study were obtained from the National Institutes of Health. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Seoul National University (SNU-220506-2).
Cell culture and differentiation
Mouse-derived C2C12 myoblasts (ATCC, VA, USA) were cultured in a growth medium containing 90% Dulbecco’s modified Eagle’s medium (DMEM; Gibco, NY, USA), 10% fetal bovine serum, and 100 unit/mL penicillin– streptomycin (PS; Gibco) at 37 °C and 5% CO2. To resolve the overcrowding phenomenon caused by cell proliferation, subcultures were performed every 48 h to maintain an appropriate number of cells. To differentiate C2C12 myoblasts into myotubes, a differentiation medium containing 98% DMEM, 2% horse serum (HS), and 100 unit/mL PS was used for a week, with the medium refreshed every alternate day, as previously described [18].
Dexamethasone (Dex) and 5-aminoimidazole-4-carboxamide-1β-4-ribofuranoside (AICAR) treatment
On the 5th day of differentiation, C2C12 myotubes were cultured in a medium containing 50% DMEM, 50% HS, and 100 units/mL PS for 2 h to induce serum shock and synchronize the periodic expression of genes. After synchronization, the cells were washed twice with 100% DMEM, and the medium was replaced with 98% DMEM, 2% HS, and 100 unit/mL PS. AICAR treatment group was treated with 1 μM AICAR (Sigma-Aldrich, MO, USA), and the Dex (Sigma-Aldrich, MO, USA) treatment group was treated with 100 nM Dex. AICAR + Dex treatment group was simultaneously treated with 1 μM AICAR and 100 nM Dex. Gene expression was measured in all four groups at 4 h intervals for 36 h.
Experimental animals and exercise
Nine-week-old male C57BL/6N mice (n = 28) were obtained from Nara Biotech Co., Ltd. (Seoul, Korea). Two weeks of acclimatization was performed using the Seoul National University Institute of Laboratory Animal Resources. Room temperature was kept constant at 21 °C and humidity was maintained at 50 ± 5%. The mice were individually housed under standard conditions with food and water provided ad libitum. All mice were divided into four groups as follows: non-stress + sedentary (NS, n = 7), nonstress + exercise (NE, n = 7), light cycle stress + sedentary (LS, n = 7), and light cycle stress + exercise (LE, n = 7) groups. An activity wheel (Tecniplast) with a 35 cm diameter and 25.8 cm width was used to exercise the mice. Body weights of the experimental animals were measured every Monday at 3:00 PM, and the number of wheel rotations in the exercise group was automatically measured by digital count and checked every Monday/Friday at 3:00 PM. For the light stress regimen, we adopted the model of Opperhuizen et al. (2017) and modified it [19]. The non-stress group was housed under a 12 h:12 h light/dark cycle, and the light cycle stress group was housed under a 14 h:10 h light (300 lx)/dark cycle for the last three weeks of the six-week experimental period. Two hours of light at night was started at 6 pm and was turned off at 8 pm. For biochemical analysis, mice were anesthetized using isoflurane. Hypothalamus and skeletal muscles were immediately frozen in liquid nitrogen and stored at −80 °C after sacrifice.
Comprehensive laboratory animal monitoring system (CLAMS)
CLAMS (Oxymax CLAMS, Columbus Instruments) is a non-invasive device used to measure the vertical and horizontal movements, food intake, drinking water, VO2 consumption, VCO2 production, and heat production of animals in real time. Room temperature was maintained at 23 °C. Of the 32 mice used in the experiments, 12 mice (three in each group) were kept for 96 h in a metabolic analysis cage. For accurate analysis, we excluded the adaptation period, which was the first 24 h. The following parameters were measured in the metabolic analysis cage: volume of oxygen consumed (VO2, mL/kg·h), volume of carbon dioxide produced (VCO2, mL/kg·h), respiratory exchange ratio (RER), heat (Cal/h), accumulated food (g), accumulated drink (g), XY total activity (all horizontal beam breaks in counts), XY ambulatory activity (minimum three different consecutive horizontal beam breaks in counts), and Z activity (all vertical beam breaks in counts). All parameters were recorded at 30 s intervals. During CLAMS analysis, all mice were housed in a single cage without voluntary wheels.
Glucose tolerance test (GTT)
For GTT, overnight-fasted mice (n = 12, 3 in each group) were intraperitoneally injected with glucose (2 g/kg) after 8 h of fasting. Blood was collected from the tail vein at 15, 30, 60, and 120 min and glucose levels were measured.
Semiquantitative RT-PCR analysis
Total RNA was extracted from the quadriceps muscles and hypothalamus of the mice using a total RNA extraction kit (RiboZol, Amresco). Then, cDNA was amplified for 15–35 cycles using gene-specific primers for mouse Bmal1, Per1, nuclear receptor subfamily 1 group D member 1 (Nr1d1), Cry, and Tbp (Table 1). Total RNA (100 ng) was amplified using PerfeCTa SYBR Green FastMix (Quanta Biosciences) and an ECO PCR system (Illumina).
Data analyses
All statistical analyses were performed using the Graph-Pad Prism 9 software (GraphPad Software Inc., San Diego, CA, USA). In cell experiments, multiple t-tests were performed to compare control vs. Dex and Dex vs. AICAR + Dex at all time points (36 h at 4 h intervals), and multiple testing corrections were not performed. A t-test was performed to compare two groups among the four groups (NS, NE, LS, and LE) in animal experiments. Comparisons between groups were performed using one-way analysis of variance forPCR analysis.
RESULTS
Body composition and blood glucose metabolism were disrupted by three weeks of light cycle stress
We designed an experiment (Figure 1) to investigate whether exercise affects light stress-induced metabolic disruption. Light is also a potent circadian stressor. Mice in the LE and LS groups were raised in a 14 h light-on state for the last three weeks of the total 6-week experimental period (Figure 1A). Mice in the NE and LE groups were given free access to a running wheel for six weeks. At the beginning of week 4, the light cycle stress group was subjected to a 14 h:10 h light/dark cycle. During the 3 weeks of light stress, the running distance of the light-cycle-stressed mice was significantly decreased compared to that of the non-stressed mice (Figure 1B). There was no significant weight variance among the groups (p > 0.05) at week 0. Body weight gain was similar (p > 0.05) in the NS and NE groups during the 6-week intervention period (Figure 1C). However, there was significant weight gain (p < 0.05) in both the LE and LS groups compared with the NS group (Figure 1C). There was no significant difference (p > 0.05) in the total food intake (data not shown) among the groups. GTT indicates the ability of the body to regulate blood sugar levels. At 60 min after glucose injection, there was a significant difference (p < 0.05) in the GTT in the NE group compared to that in the other groups (Figure 1D). In the absence of stress, exercise reduces blood glucose levels at specific time points. However, at 90 min, there was a significant difference (p < 0.05) only in the LS group compared with the NE group (Figure 1D). There were no significant differences (p > 0.05) among the groups at the other time points (0, 30, and 120 min). Similarly, the glucose area under the curve during the GTT was not significantly different between the groups. VO2 consumption and heat production increased during the night cycle but not under light cycle stress conditions
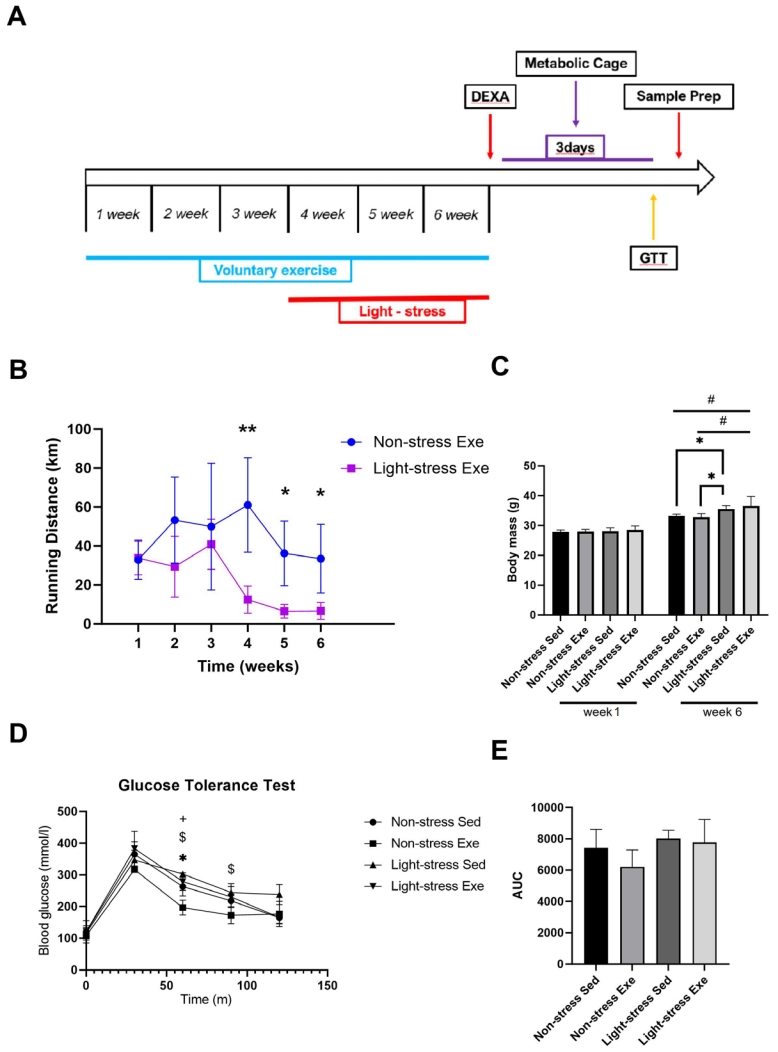
(A) Experimental setup. Male C57BL/6 mice (n = 28), aged 9 weeks, were randomly assigned to a voluntary wheel running (E, n = 14) or sedentary (S, n = 14) group. (B) Voluntary wheel running (VWR) distance was continuously monitored using a tachometer in single-housed mice. Mean distance (per week) over time (n = 7, respectively) is shown for VWR mice. (C) Effect of light stress and/or exercise on body weight. Data represent the mean ± standard deviation. *p<0.05, **p<0.01, and #p<0.05 (D) Glucose response and (E) area under the curve (AUC) values for a glucose tolerance test (GTT). Data represent the mean ± standard deviation. *p<0.05, NS vs. NE; +p<0.05, NS vs. LE; $p<0.05, NS vs. LS.
We assessed the differences in metabolic parameters among the groups using metabolic chambers. Distinct pattern changes were observed in the dark:light cycles of all groups (Figure 2A). Separate analyses of each phase showed that the RER level was significantly lower (p < 0.05) in the LS group than in the NS group during the dark phase (Figure 2A). However, this stress effect was blunted (p > 0.05) in the LE group compared to that in the NS group (Figure 2A). In the dark cycle, there was a significant increase in the x-axis activity compared to that in the light cycle in both the NS (p < 0.001) and NE (p < 0.05) groups, but there was no significant difference between the dark and light cycles (p > 0.05) in both the LS and LE groups (Figure 2B). However, there was no significant difference (p > 0.05) in the total RER during the dark cycle or day in the metabolic chambers among the groups (Figure 2C). In the dark cycle, there was a significant increase in VO2 consumption (p < 0.05) compared with that in the light cycle in both the NS and NE groups, but there was no significant difference between the dark and light cycles (p > 0.05) in both the LS and LE groups (Figure 2D). Similarly, heat production also significantly increased in the dark cycle in the NS and NE groups compared to that in the light cycle, but not in the LS and LE groups (Figure 2E). These data imply that three weeks of light cycle stress was sufficient to inactivate metabolic parameters, even in voluntarily exercised mice.
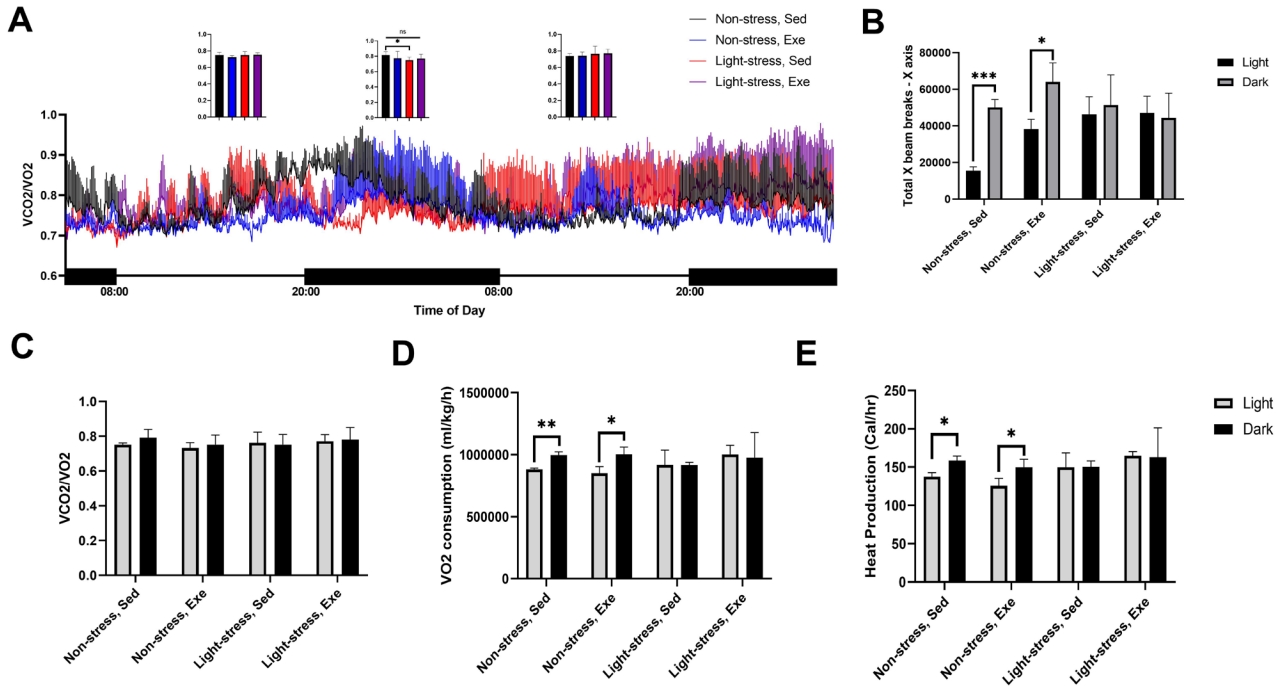
Effect of exercise on the metabolic rate of light cycle-stressed mice. (A) Respiratory exchange ratios (RERs) in the exercise and light stressed mice groups. Small graphs comparing the RERs between groups in each phase. (B) Average ambulatory activity in the X-axis during light and dark cycles (C) Average value of 3-day recorded RERs in light and dark cycles. (D) VO2 consumption in exercise and light stressed mice groups in light and dark cycle. (E) Heat production in exercise and light stressed mice groups during light and dark cycles. Values are expressed as the mean ± deviation obtain from three mice. *p ≤ 0.05.
Voluntary exercise partially recovers Per1 expression disrupted by light cycle stress in quadriceps muscles
Expression levels of circadian genes are shown in Figure 3. The hypothalamus is responsible for synchronizing internal rhythms. To examine the effect of exercise on circadian-related gene fluctuations induced by light-cycle stress, we measured the mRNA expression levels of Baml1, Per1, Cry1, and Nr1d1 in the hypothalamus. There were no significant differences in hypothalamic gene expression between the groups. Circadian genes within the skeletal muscles also play critical roles in the regulation of energy metabolism [20]. To reveal how exercise and light cycle stress influence circadian gene expression in skeletal muscles, we compared the relative mRNA expression levels of Baml1, Per1, Cry1, and Nr1d1 in quadriceps muscles. Per1 expression was significantly reduced in the quadriceps muscles of the LS group compared to that in the muscles of the NS group. However, there was no significant difference between the NS and NE or LE groups. These results underscore the possibility that exercise partially affects the light cycle stress-interrupted homeostasis of circadian-related transcription in skeletal muscles.

Circadian gene expression levels in the hypothalamus (HYP) and quadriceps (QUAD) muscles of control and exercise mice after exposure to light stress. Changes in the relative mRNA expression levels of basic helix-loop-helix ARNT-like 1 (Baml1) (A, E), period circadian regulator 1 (Per1) (B, F), cryptochrome 1 (Cry1) (C, G), and nuclear receptor subfamily 1 group D member 1 (Nr1d1) (D, H). Amount of mRNA in each sample was normalized to the factor, TATA box-binding protein (Tbp), of the sample. Values are expressed as the arithmetical mean ± standard deviation. n = 4. p ≤ 0.05; ns: not significant.
Exercise mimetics moderately affect the circadian gene oscillations induced by light cycle stress mimetics in differentiated myotube cell lines
High serum concentrations induce the circadian expression of various genes whose transcription also oscillates in living animals [21]. In our experimental settings, differentiated C2C12 cells were subjected to the same regimen of serum treatment, and the levels of several mRNAs were monitored after treatment with an exercise mimetic (AICAR), a stress hormone mimetic (Dex), or both AICAR and Dex (AICAR + Dex). Comparative analysis showed that Bmal1 expression was significantly higher in the AICAR + Dex treatment group than in the control group at 16 h (Figure 4A). The mRNA expression level of Per1 was altered in the AICAR + Dex treatment compared to that of the control at 12 and 24 h, whereas Dex treatment alone significantly downregulated compared to that of the control at 16 h (Figure 4B). At 20 and 24 h, the mRNA level of Cry1 was significantly reduced in both the Dex-only and AICAR + Dex treatments compared to that in the control. However, at the 28 h time point, the expression level of Cry1 only changed in the Dex treatment compared to that in the control (Figure 4C). Compared to the control, the mRNA expression levels of Nr1d1 were upregulated in the Dex treatment group at 24 and 28 h (Figure 4D). Overall, these results imply that exercise-induced AMP-activated protein kinase (AMPK) activation could not fully reverse the effects of stress, but is a potential regulator that plays an important role in circadian gene oscillation disrupted by light cycle stress mimetic conditions.

Oscillation of the expression of circadian clock genes in C2C12 skeletal myotubes. Black line indicates the differentiated C2C12 cells treated with the vehicle (control); red line indicates the differentiated C2C12 cells treated with 1 µM Dex; purple line indicates the differentiated C2C12 cells co-treated with 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR) and dexamethasone (Dex). Cells were collected after 0, 6, 12, 18, 24, and 30 h for RNA extraction. Gene expression was analyzed using quantitative polymerase chain reaction (qPCR) of Bmal1 (A), Per1 (B), Cry1 (C), and Nr1d1 (D). Each experiment was performed in triplicate. Data represent the mean ± standard deviation. *p<0.05, CON vs. Dex; #p<0.05, CON vs. AICAR+Dex; Dex, dexamethasone.
DISCUSSION
In this study, we provide evidence that skeletal muscle circadian rhythm is regulated by voluntary exercise, resulting in partial amelioration of the metabolic burden in an aberrant light exposure rodent model. The results confirmed that in the quadriceps muscles, the reduction in Per1, a circadian gene, induced by three weeks of light stress, could be improved by voluntary wheel exercise.
Circadian disruption by nighttime light perturbations is associated with an increased risk of metabolic dysfunction [22,23], although it tends to differ in nocturnal mice [24]. Circadian genes are important regulators of glucose and lipid metabolisms [11]. We also observed that light cycle stress increased body mass in both the stress and stress with exercise groups. Voluntary running distance also decreased after the light cycle stress, which may have affected the reduction in VO2 consumption and heat production during the night cycle. Voluntary wheel running in mice elevated basal activity in the dark cycle during metabolic cage analysis but not in the light stress group. Nevertheless, according to the GTT results, voluntary exercise had a slight effect on the recovery of damaged glucose metabolism in mice subjected to light cycle stress. Bilu et al. [25] showed that exercise improved the metabolic state in a diurnal model of circadian disruption [25]. However, this result was not observed in the experimental model. One reason for this may be reduced exercise volume due to light cycle stress (Figure 1B).
In mammals, the SCN regulates the circadian clock in mammals [11]. Light signaling induces rapid changes in SCN cellular activity Exposure to chronically low levels of light at night alters the circadian clock genes in both the SCN and peripheral tissues of mice [26,27]. In rodent experiments, light-cycle stress suppressed Per1 expression in the SCN of the hypothalamus around the light/dark transition, whereas the expression levels of Bmal and Cry were unaffected by lighting conditions [27]. The skeletal muscle system has its own clock gene expression that can be stimulated by physical activity. Acute session (70% of VO2 max) of aerobic exercise increases the expression of skeletal muscle clock genes, such as BMAL1, in trained men [28]. In a rodent model, chronic (four weeks) low-intensity resistance exercise resulted in a significant change in the expression of clock genes in the skeletal muscles of mice [29]. These results indicate that exercise could be a potent cue to modulate the circadian rhythm in peripheral tissues, such as skeletal muscles. However, few studies have investigated the effects of photic stimulation on the oscillations of circadian genes in skeletal muscles. In our experimental model, an aberrant light schedule significantly attenuated the expression of Per1 in skeletal muscle, but not in the hypothalamus. Mice deficient in Per1/2/3 exhibit weight gain following a high-fat diet [30]. Although we could not prove causality, we confirmed weight gain under light cycle stress conditions. We did not observe any significant changes in Bmal1, Cry1, and Nr1d1 expression by exercise or light cycle stress in either the quadriceps muscles or hypothalamus, possibly because of the limited time points.
A short light pulse administered to rodents at night increased plasma corticosterone levels [31]. Increased corticosterone levels during acute stress episodes efficiently suppress the appetite associated with weight loss [32]. In contrast, chronic stress with high corticosterone levels increases appetite, resulting in hyperglycemia and insulin resistance [33]. Circadian genes in the peripheral tissues regulate lipolytic triglyceride breakdown and fatty acid release, thereby affecting appetite [34,35]. In this study, we investigated whether circadian gene perturbation by stress hormones is regulated by exercise-induced intramuscular enzymes. AMPK is a proposed signaling mediator that affects exercise and circadian rhythms in skeletal muscles [36]. Using the AMPK activator AICAR, many groups have demonstrated the underlying mechanism of action of exercise in cellular models [13,37]. Here, we suggest that though AMPK activity does not represent all exercise signals, AMPK activation plays an important role in maintaining the oscillation of circadian disruption induced by stress hormones. Therefore, other mechanisms may be involved in maintaining circadian homeostasis during exercise.
Prevalence of night-time work has increased drastically in recent years. In humans, acute and chronic changes in the light regimen have different effects on different variables, depending on their regulation [38,39]. Disturbances in the circadian system are considered as the pathogenic mechanisms of metabolic diseases [40]. In this study, we found that voluntary exercise partially prevented artificial light-cycle stress-induced metabolic challenges, possibly by regulating the peripheral circadian genes in mice. Therefore, we should consider the beneficial effects of exercise in rescuing the light-induced disruptions of circadian rhythms, which may vary between nocturnal and diurnal species and are more prominent in humans than in nocturnal animals [41]. Furthermore, AMPK activation may mediate the metabolic benefits of exercise by regulating circadian gene expression. Our findings indicate the partial preventive effects of exercise on metabolic disruptions via circadian gene modulation in skeletal muscles.
Acknowledgements
H.M. and I.J. designed the study, analyzed the data, and wrote the first draft of the manuscript.
We are grateful to all the individuals who kindly agreed to participate in this study.
This study was funded by the National Research Foundation (NRF) of Korea (NRF-2020R1C1C1006414 and NRF-2022R1I1A4053049) and supported by an NRF grant funded by the Korean government (MSIT; No. 2022R1A5A8019303).
The authors declare that they have no competing financial interests or personal relationships that may have influenced the work reported in this study.
