Effect of long-term carnosine/anserine supplementation on iron regulation after a prolonged running session
Article information
Abstract
[Purpose]
Exercise-induced hemolysis, which is caused by metabolic and/or mechanical stress during exercise, is considered a potential factor for upregulating hepcidin. Intramuscular carnosine has multiple effects including antioxidant activity. Therefore, this study aimed to determine whether long-term carnosine/anserine supplementation modulates exercise-induced hemolysis and subsequent hepcidin elevation.
[Methods]
Seventeen healthy male participants were allocated to two different groups: participants consuming 1,500 mg/day of carnosine/anserine supplements (n = 9, C+A group) and participants consuming placebo powder supplements (n = 8, PLA group). The participants consumed carnosine/anserine or placebo supplements daily for 30.7 ± 0.4 days. They performed an 80-running session at 70% V̇O2peak pre-and post-supplementation. Iron regulation and inflammation in response to exercise were evaluated.
[Results]
Serum iron concentrations significantly increased after exercise (p < 0.01) and serum haptoglobin concentrations decreased after exercise in both groups (p < 0.01). No significant differences in these variables were observed between pre-and post-supplementation. Serum hepcidin concentration significantly increased 180 min after exercise in both groups (p < 0.01). The integrated area under the curve of hepcidin significantly decreased after supplementation (p = 0.011) but did not vary between the C+A and PLA groups.
[Conclusion]
Long-term carnosine/anserine supplementation does not affect iron metabolism after a single endurance exercise session.
INTRODUCTION
Iron status is frequently impaired due to iron loss from sweating, hemolysis (i.e., disruption of red blood cells), gastrointestinal bleeding [1-3] during physical training, or inadequate iron intake from the daily diet [4]. Athletes present a higher prevalence of iron deficiency, with 15-35% of female athletes and 3-11% of male athletes [5,6]. Koehler et al. (2012) [7] reported that 30% of male athletes showed a suboptimal iron status (serum ferritin concentration < 35ng/mL) although 81% of male athletes satisfied the recommended dietary allowance criteria for elemental iron. Attenuated iron metabolism due to hepcidin, a liver-derived peptide hormone, contributes to iron deficiency in athletes [8].
Hepcidin regulates iron metabolism by affecting the only known iron-exporter protein, ferroportin [9,10]. Hepcidin hampers iron absorption in the duodenum and iron recycling from the macrophages via the occlusions or endocytosis of ferroportin [11]. Hepcidin gene expression is regulated in response to intracellular and/or extracellular iron levels (e.g., Fe2-transferrin concentration and iron stores in the liver) and interleukin-6 (IL-6) due to systemic inflammation [12]. Oral iron supplementation has been reported to negatively affect iron metabolism by augmenting hepcidin levels, as oral iron supplementation temporarily increases circulating iron levels [13-15]. Moretti et al. (2015) [14] showed that the consumption of oral iron doses ≥ 60 mg (60, 80, 160, 240 mg) significantly increased plasma hepcidin concentration within 24 h, with a 35–45% subsequent reduction in iron absorption. Ishibashi et al. (2017) [13] demonstrated increased basal hepcidin concentrations during three consecutive days of an endurance training block with 24 mg of iron supplementation daily. Altogether, excessive oral iron supplementation appears to attenuate iron absorption via elevated hepcidin concentration. Circulating hepcidin levels also increase after an acute session of prolonged exercise, which is thought to involve elevated IL-6 and hemolysis [8,16]. Serum and urine hepcidin concentrations are generally maximized approximately 3–6 h after completing exercise when a transit window of attenuated iron metabolism exists [17,18]. Therefore, alternative nutritional approaches to iron supplementation are required to prevent iron deficiency in athletes.
Carnosine (β-alanyl-L-histidine) and anserine (β-alanyl-1-meth-yl-L-histidine) are imidazole dipeptides. L-histidine and β-alanine are supplied from carnosine and/or anserine in the diet and are synthesized as carnosine in the skeletal muscle. In previous studies, 4 weeks of β-alanine supplementation significantly increased the muscle carnosine concentration [19,20]. Intramuscular carnosine has multiple effects, such as antioxidant activity, buffering capacity, calcium sensitivity, and metal chelation [21,22], which affect local hemostasis and contribute to homeostasis in other organs by acting on target cells through its release into the blood during muscle contraction (i.e., physical activity and exercise) [23]. Exercise, particularly running, causes hemolysis, leading to increased circulating iron and/or decreased haptoglobin levels [24,25]. Exercise-induced hemolysis is considered a potential upregulating factor for the post-exercise hepcidin response [8], which is attributed to two different mechanisms: mechanical injury (e.g., foot impact, muscle contraction, and vasoconstriction) and metabolic injury (e.g., hyperthermia, lactic acidosis, shear stress, and oxidative damage) [26]. Accumulation of carnosine in skeletal muscle with carnosine/anserine supplementation may attenuate metabolic injury [26] since intramuscular carnosine has an antioxidative capacity and/or buffering capacity, consequently reducing increased erythrocyte fragility and suppressing exercise-induced hemolysis. Moreover, changes in Fe2-transferrin concentrations due to the attenuation of exercise-induced hemolysis may modulate hepcidin secretion [11].
Therefore, the present study investigated the effects of long-term supplementation of carnosine/anserine on iron regulation following a single session of endurance running. We hypothesized that carnosine/anserine supplementation would attenuate exercise-induced hemolysis and the subsequent hepcidin elevation.
METHODS
Participants
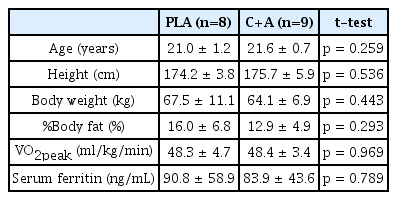
The present study included seventeen active males [mean ± standard deviation (SD), age: 21.3 ± 1.0 years, height: 175.0 ± 4.9 cm, body weight (BW): 65.7 ± 9.0 kg, peak oxygen uptake (V̇O2peak): 48.3 ± 4.0 mL/kg/min)]. All participants were informed of the purpose of the study, the experimental protocol, and the possible risks involved. Written informed consent was obtained from all participants. This study was approved by the Ethics Committee for Human Experiments at Ritsumeikan University (BKC-2018-046) in accordance with the Declaration of Helsinki.
Group Classification
The participants were assigned to two different groups: participants consuming 1,500 mg (500 mg, three times) of carnosine/anserine supplementation (C+A group; n = 9) or participants consuming 1,500 mg of placebo (PLA group; n = 8; Table 1). Participants were instructed to refrain from strenuous activity, consume supplements for at least 48 h before testing, and not consume alcohol for at least 24 h before testing. Consumption of additional supplements was restricted during the experimental period.
Experimental Overview
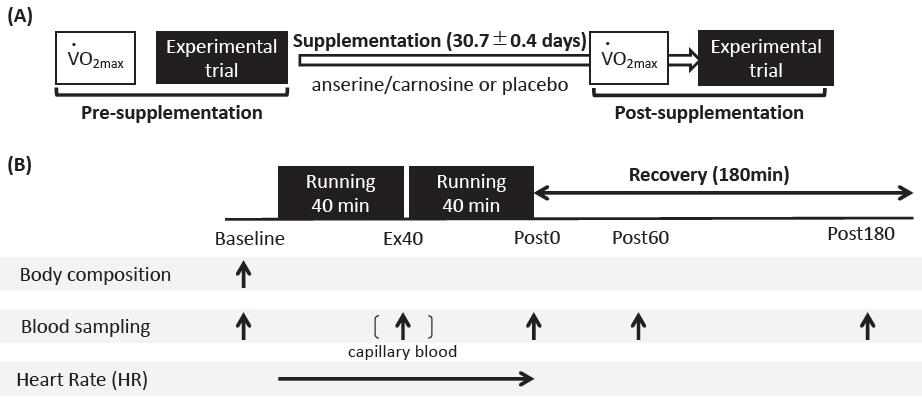
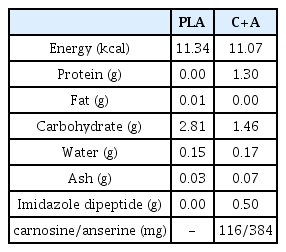
The participants visited our laboratory four times for the two graded running tests (pre- and post-supplementation) and two experimental trials (pre- and post-supplementation) throughout the experimental period (Figure 1A). At the first visit, the V̇O2peak was evaluated using a graded running test to determine the relative running velocity (70% V4 O2peak) for the main pre-supplementation trial. On the second visit, an 80-min running task (pre-supplementation experimental trial) was performed. After completion of the experimental trial, participants started consuming either carnosine/anserine or placebo powder supplements three times a day until the day before the secondary experimental trial (the average consumed period was 30.7 ± 0.4 days). Each pack of carnosine/anserine supplements contained 500 mg of imidazole dipeptide (carnosine 384 mg, anserine 116 mg); thus, participants in the carnosine/anserine group consumed a total of 1,500 mg carnosine/anserine per day. Powdered supplements were manufactured by NH Foods Ltd. (Osaka, Japan) (Table 2). On the third visit, V̇O2peak was reevaluated 1–3 days before the secondary experimental trial after supplementation in both groups. The participants were required to avoid supplement intake 2-h before the graded running test. On the fourth visit, an 80-min running task (post-supplementation experimental trial) was performed. Iron metabolism and inflammation were compared after the running task pre-and post-supplementation.
Graded Exercise Test
A graded exercise test was performed on a treadmill (Valiant, Lode B. V., Groningen, Netherlands) to determine V̇O2peak. The treadmill gradient was set to 0% during the test. Following a 3 min warm-up at 6 km/h, the initial running velocity was set at 10 km/h and progressively increased by 2 km/h every 2 min. When the running velocity reached 14 km/h, it increased by 0.6 km/h every 1 min until voluntary exhaustion. Respiratory gas was collected throughout the testing session using the breath-by-breath method with an automatic gas analyzer (AE300S; Minato Medical Science, Tokyo, Japan) to determine the V̇O2peak. Data for respiratory variables (V4 O2 and V4 CO2peak) were measured on average every 30 s.
Experimental Trials
Experimental trials consisted of an 80-min running exercise at 70% V̇O2peak followed by a series of blood samplings during post-exercise (Figure 1B). The experimental trials were conducted twice before and after supplementation. On the respective days, the participants arrived at the laboratory (8:00 am) following an overnight fast (> 10 h), and data on body composition and blood samples (baseline) were initially collected. After collecting the initial blood samples, the participants performed 80 min of exercise at 70% V̇O2peak. The exercise consisted of two 40-minute running sessions and 3-min of passive rest between sessions. During the 3 min passive rest, a blood sample was collected from the fingertip to measure blood glucose and lactate concentrations (Ex40). After completion of the exercise, further blood samples were collected immediately after exercise (Post0), 60 min after exercise (Post60), and 180 min after exercise (Post180). The heart rate (HR) was recorded every 60 s using a wireless HR monitor (RCX5, Polar, Tokyo, Japan).
Blood Sampling and Analysis
Blood samples were collected from the antecubital vein before, immediately after, and 60 and 180 min after exercise using serum-separating tubes and tubes containing Na2-ethylenediaminetetraacetic acid (EDTA). Blood samples were centrifuged at 3,000 rpm and 4℃ for 10 min for serum or plasma separation. Obtained serum and plasma were stored at −60℃ until analysis. Blood glucose and lactate concentrations were measured immediately after blood sampling using a glucose analyzer (Free Style; Nipro Corporation, Osaka, Japan) and a lactate analyzer (Lactate Pro; Arkray, Kyoto, Japan), respectively. The concentrations of ferritin, iron, myoglobin (Mb), haptoglobin (Hp), and hepcidin were determined. Serum ferritin, iron, Mb, and Hp concentrations were measured at a clinical laboratory (SRL, Tokyo, Japan). Serum hepcidin concentrations were measured using an enzyme-linked immunosorbent assay (ELISA) with a commercially available kit (R&D Systems, Minneapolis, MN, USA) The intra-assay coefficient of variation (CV) was 1.9, 2.3, 1.6, 2.3, and 2.2%, respectively. From the obtained plasma samples, IL-6 concentration was also measured using ELISA with a commercially available kit (R&D Systems). The intraassay CV values were 2.2, 1.7, and 1.4%, respectively.
Statistical analysis
Statistical analyses were performed using JASP version 0.16.2 [Computer software] (JASP Team, 2020). An Independent t-test was used to compare participant characteristics between the two groups. A mixed analysis of variance ANOVA was used to analyze a trial effect (pre- and post-intervention), a group effect (C+A, PLA), and an interaction between these two factors on the changes in running velocity, HR, and blood lactate response, baseline iron status (ferritin and iron), and area under the curve (AUC) of iron, haptoglobin, and hepcidin. AUCs of hepcidin, iron, and haptoglobin concentrations were calculated using the trapezoid area method at each time point (Baseline, Post0, Post60, Post180). Two-way repeated measures ANOVA within subjects was applied to confirm a time effect (Baseline, Post0, Post60, Post180), a trial effect (pre, post), and interaction (trial × time) to compare the differences in plasma IL-6, serum Mb, Hp, iron and hepcidin responses before and after supplementation. When significant interactions or main effects were observed, post hoc Bonferroni correction was applied for post hoc comparison to determine where specific differences existed. Effect sizes (ES) were summarized as partial eta squared (ηp2) for ANOVA and as Cohen’s d for post hoc Bonferroni correction. ES of d = 0.2 is classified as “small,” d = 0.5 is as “medium,” and d = 0.8 is “large [27].” All data are presented as means ± standard deviation (SD) and the statistical significance level was accepted at p < 0.05.
RESULTS
Aerobic capacity
No main time effect (p = 0.077, ηp2 = 0.194), group (p = 0.787, ηp2 = 0.005), or interaction (p = 0.304, ηp2 = 0.070) was reported for V̇O2peak (PLA group: pre 48.3 ± 4.7 ml/kg/min, post 48.6 ± 4.6 ml/kg/min; C+A group: pre 48.4 ± 3.4 ml/kg/min, post 49.6 ± 2.7 ml/kg/min). A main trial effect (p < 0.001, ηp2 = 0.665) was observed for running velocity during experimental trials. Running velocity significantly increased post-supplementation compared to pre-supplementation in both groups (PLA group: pre 10.5 ± 0.7 km/h, post 10.9 ± 0.7 km/h; C+A group: pre 10.7 ± 0.7 km/h, post 11.2 ± 0.7 km/h; p < 0.001, d = -0.607).
Iron status
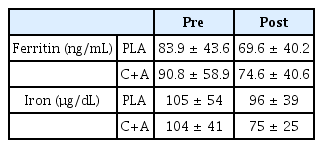
Baseline serum ferritin concentrations did not show any interaction (p = 0.924, ηp2 = 0.000), trial (p = 0.141, ηp2 = 0.139), or group effects (p = 0.775, ηp2 = 0.006, Table 3). Baseline serum iron concentration did not present any interaction (p = 0.376, ηp2 = 0.053) or group effects (p = 0.518, ηp2 = 0.028), but showed a tendency toward a trial effect (p = 0.087, ηp2 = 0.183, Table 3).
HR and Blood Lactate Response
Heart rate as of the end of the exercise did not present any interaction (p = 0.277, ηp2 = 0.078), trial (p = 0.168, ηp2 = 0.123) and group effects (p = 0.866, ηp2 = 0.002; PLA group: pre 169 ± 14 bpm, post 168 ± 15.9 bpm; C+A group: pre 172 ± 10 bpm, post 167.4 ± 10 bpm). Blood lactate concentration immediately after exercise did not present any interaction (p = 0.236; ηp2 = 0.092), trial (p = 0.659, ηp2 = 0.013) and group effect (p = 0.236; ηp2= 0.092; PLA group: pre 1.9 ± 0.6 mmol/L, post 2.0 ± 1.0 mmol/L; C+A group; pre 2.5 ± 0.7 mmol/L, post 2.2 ± 0.7 mmol/L).
Hemolysis and Iron regulation
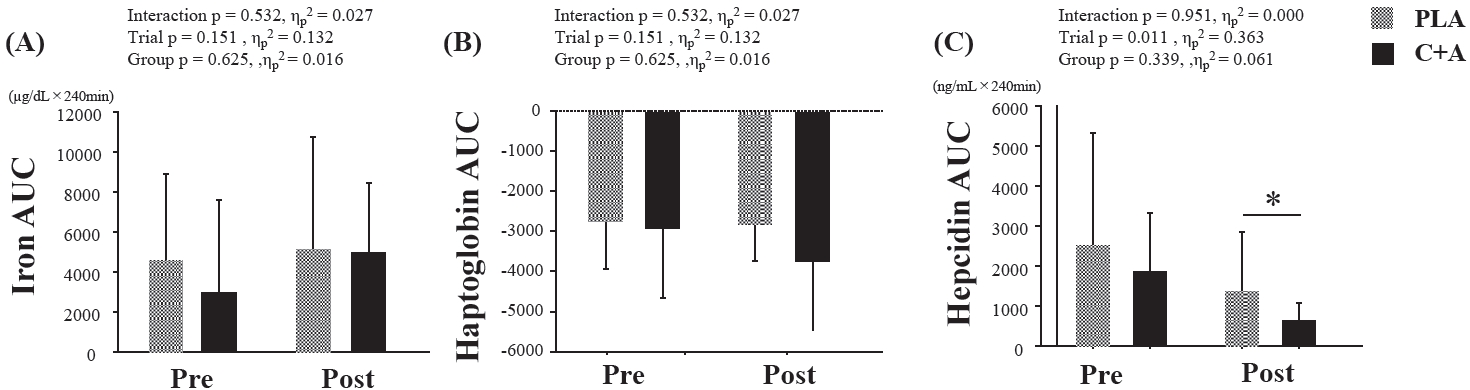
Serum Hp concentrations in the PLA group significantly decreased after exercise (Post0, p = 0.001, d = 0.171; Post60, p < 0.001, d = 0.419; Post180, p < 0.001, d = 0.334), with no difference between pre-and post-supplementation (Figure 2). Serum Hp concentrations in the C+A group also significantly decreased after exercise (Post0, p = 0.002, d = 0.226; Post60, p < 0.001, d = 0.559; Post180, p < 0.001, d = 0.56), with no difference pre-and post-supplementation. Serum iron concentrations in the PLA group significantly increased after exercise (Post0, p < 0.001, d = -0.496; Post60, p = 0.002, d = -0.456; Post180, p < 0.001, d = -0.487) with no difference pre- and post-supplementation. Serum iron concentrations in the C+A group also significantly increased after exercise (Post0, p = 0.001, d = -0.503; Post60, p = 0.005, d = -0.443; Post180, p < 0.001, d = -0.556), with no difference pre-and post-supplementation (Figure 2). Serum hepcidin concentrations significantly increased 180 min after exercise in both the PLA and C+A groups (p = 0.004, d = -1.03; p < 0.001, d = -0.811, respectively), and there was no significant difference in hepcidin responses between preand post-supplementation (Figure 2).
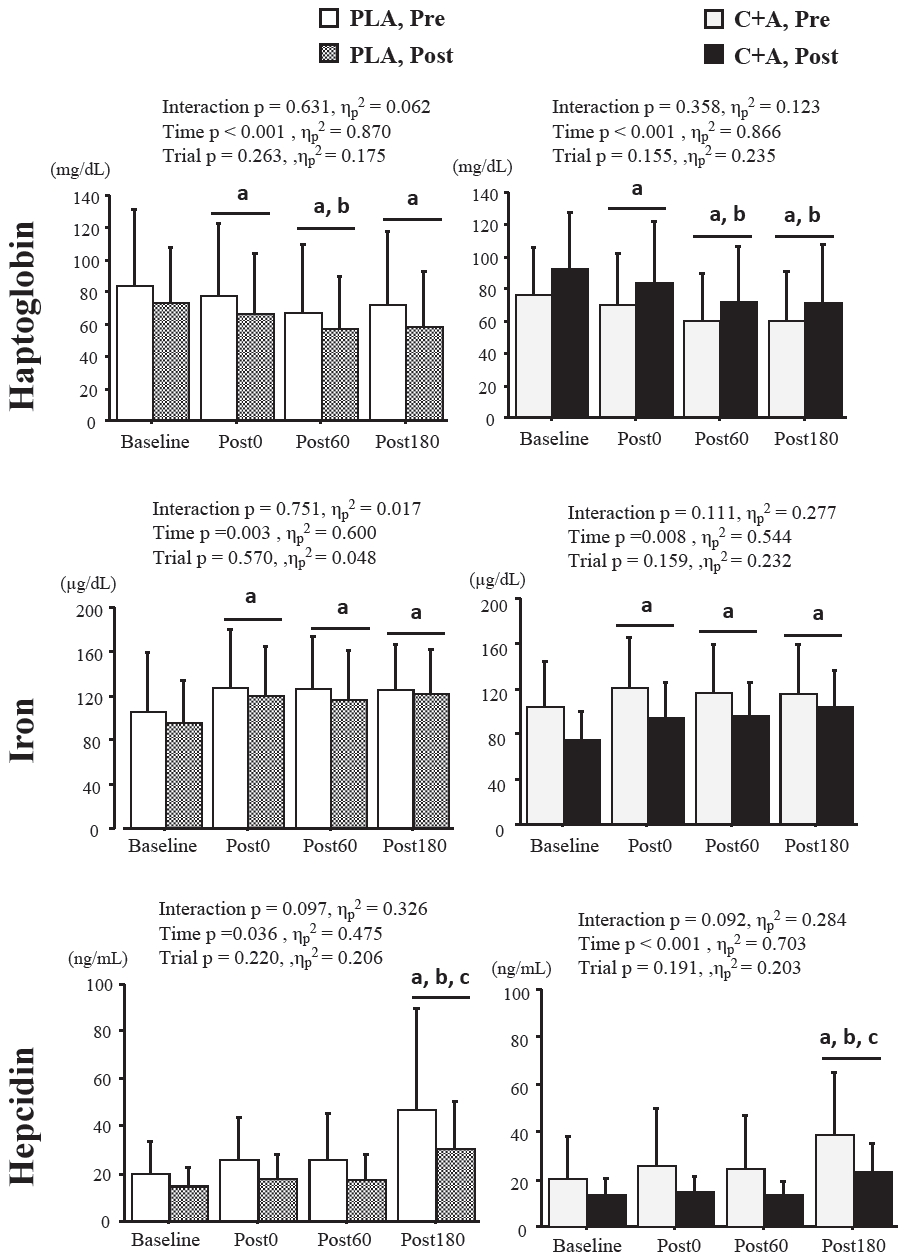
Changes in serum haptoglobin, iron, and hepcidin response pre-and post-supplementation. Values are presented as means ± SD. Pre, pre-supplementation; Post, post-supplementation; PLA, placebo group; C+A, carnosine/anserine group, Post0, a point immediately after exercise; Post60, a point of 60 min after exercise, Post180, a point of 180 min after exercise. a Significant difference compared to baseline, b Significant difference compared to P0, c Significant difference compared to Post60
The iron AUC and Hp AUC did not present any interaction, trial, or group effects (Figure 3). The hepcidin AUC significantly decreased post-supplementation (p = 0.011, d = 0.696), but did not vary between the groups (Figure 3).
Muscle Damage and Inflammation
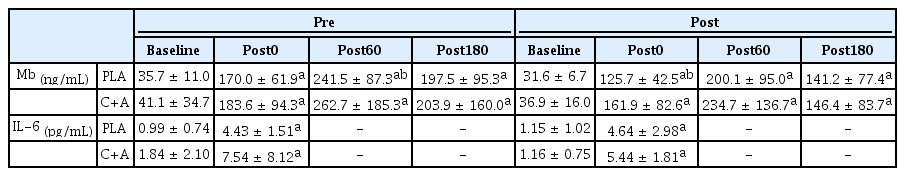
Serum Mb concentration showed a time effect in both the PLA (p < 0.01, ηp2 = 0.846) and C+A group (p = 0.003, ηp2 = 0.654). The serum Mb concentration in the PLA group significantly increased after exercise (Post0: p < 0.001, d = -1.667; Post60: p < 0.001, d = -2.732; Post180: p < 0.001, d = -1.981), but did not differ pre- and post-supplementation (Table 4). Serum Mb concentration in the C+A group also significantly increased after exercise (Post0: p = 0.002, d = -1.180; Post60: p < 0.001, d = -1.850; Post180: p = 0.002, d = -1.201), but did not vary pre- and post-supplementation (Table 4).
Plasma IL-6 concentrations in the PLA group showed a time effect (p = 0.003, ηp2 = 0.740). The IL-6 concentration in the PLA group immediately after exercise significantly increased (p = 0.003, d = -1.940) but did not differ between pre- and post-supplementation (Table 4). Plasma IL-6 concentration in the C+A group also showed a time effect (p = 0.002, ηp2 = 0.720). Plasma IL-6 concentration in the C+A group also significantly increased immediately after exercise (p = 0.002, d = -1.159) but did not differ between pre- and post-supplementation (Table 4).
DISCUSSION
This study investigated the influence of long-term carnosine/anserine supplementation on post-exercise iron regulation using a single-blind, placebo-controlled test. No significant differences in hemolysis, inflammation, or muscle damage were observed following the 80-min running between pre- and post-supplementation (30.7 ± 0.4 days). Exercise-induced hepcidin elevation appeared to be modulated after supplementation in both groups; however, no apparent effect of carnosine/anserine on exercise-induced hepcidin elevation was observed.
Although recent studies have reported the effects of anti-inflammatory or antioxidant supplements and foods on exercise-induced hepcidin responses, no significant effects on elevated hepcidin concentrations after exercise have been observed regardless of the duration or timing of intake. A study that added vitamin D (5,000 IU) and K (1,000 μg) to mixed carbohydrate and protein intake after high-intensity interval cycling exercise demonstrated that no effect of supplementation on the post-exercise iron status or IL-6 and hepcidin concentrations [28]. Long-term 28-day intake of both vitamin C (500 mg/day) and vitamin E (400 IU/day) did not affect hepcidin concentration after 90 min of running exercise [29]. The supplementation of polyphenols, which have antioxidant properties, is also expected to reduce oxidative stress, leading to improving iron metabolism [12,30]. The consumption of chokeberry juice (50 ml, three times a day) during an 8-week training camp for rowers increased their antioxidant capacity and attenuated an increase in TNF-α concentrations 24 h after a 2,000 min time trial. However, no effect on IL-6 or hepcidin response was observed after the trial [30]. In addition, supplementation with cranberry extract for 6 weeks enhanced the antioxidant capacity associated with high-intensity exercise, while no clear effects on IL-6, Mb, and hepcidin responses were observed12. The results of this study align with those of the aforementioned studies.
An increased serum iron concentration with a concomitant reduction in serum Hp concentration was observed in this study, which is a typical symptom of exercise-induced hemolysis [24,25]. Accordingly, hemolysis would occur after the endurance running session; however, the magnitude of exercise-induced hemolysis was independent of carnosine/anserine or placebo supplementation because of similar responses in serum iron and Hp concentrations. The baseline iron status (i.e., serum iron and ferritin concentrations) was slightly different between pre-and post-supplementation in the C+A group; however, these differences did not reach statistical difference. A slight decrease in baseline serum iron (PLA group -9.1%; C+A group -27.7%) and ferritin (PLA group, -17.0%, C+A group -17.8%) may affect the post-exercise hepcidin response. Peeling et al. (2017) [31] also showed that both basal serum iron and ferritin concentrations significantly correlated with serum hepcidin concentration 3 h after exercise. Therefore, changes in baseline iron status would modulate iron regulation in response to the running exercise and, as a result, would have lowered the AUC of the hepcidin concentration post-supplementation. L-Histidine, a component of carnosine and anserine, may also be involved in the reduction of the AUC of serum hepcidin concentrations; it is involved in erythropoiesis and is thought to be related to iron absorption [32,33]. It is an essential amino acid for erythrocyte production [34]. Therefore, extra- and intracellular iron may be mobilized for hematopoiesis, leading to a slight decrease in ferritin and serum iron levels and a subsequent attenuated hepcidin response.
The increase in IL-6 concentration immediately after exercise is thought to be a potential factor that induces 3h post-exercise hepcidin elevation via the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway [35]. Running sessions for 80 min significantly increased the plasma IL-6 concentration, and this response did not change post-supplementation. The hepcidin response post-supplementation was lower than that pre-supplementation in both groups, indicating that modification of the IL-6– hepcidin axis was not a primary factor for the attenuation of hepcidin concentration. A previous clinical study reported lower IL-6 concentrations after 12 weeks of l-carnosine supplementation in patients with type 2 diabetes [36]. In addition, carnosine supplementation attenuated IL-6 secretion by activated murine macrophages in vitro [37]. Increased IL-6 concentration immediately after exercise can be differentiated as muscle-derived IL-6 [38], indicating that although carnosine/anserine intake may downregulate the increase in macrophage-derived IL-6 production due to chronic inflammation, it does not influence muscle-derived IL-6. Although the relative load (70%V4 O2peak) was standardized pre- and post-supplementation in this study, the absolute load (i.e., running speed) post-supplementation was not equivalent to that pre-supplementation because of the slight increase in V̇O2peak. Since exercise-induced IL-6 is influenced by exercise modality, duration, and intensity [39,40], caution is needed when interpreting the results when absolute running speed differs. However, the difference in speed pre- and post-supplementation in this study was ~0.4-0.5 km/h, suggesting that it is unlikely to affect IL-6 production.
A major limitation of the present study was the lack of detailed data on energy intake and macro- and micronutrient intake, as no dietary survey was conducted during the supplementation period; therefore, dietary data would be helpful to understand the effects of carnosine/anserine supplementation more fully. All participants were instructed to maintain their daily activities and regular diets during the supplementation period, and no significant changes in body composition were observed pre- and post-supplementation. A previous study demonstrated that the 30-day ingestion of chicken breast extract (CEBX) containing 1,500 mg of carnosine/anserine significantly increased intramuscular carnosine concentration in the vastus lateralis muscle in healthy men [41]. Because the C+A group consumed the same amount of carnosine/anserine as Sato et al. (2003) [41] for approximately 30 days, apart from their diet, it was assumed that the intramuscular carnosine concentration also increased in this study as well.
The participants, in this study, did not conduct any structured exercise training during the supplementation period; therefore, the effects of carnosine/anserine in combination with exercise training on iron metabolism are unclear. Zügel et al. (2020) [42] showed an increase in hepcidin concentrations with increasing amounts of high-intensity training, indicating that the regulation of hepcidin secretion is sensitive to changes in training load. In athletes, exercise stimuli may affect iron regulation [43], and metabolic stress-induced hemolysis during exercise has been reported to vary with subject characteristics (fitness level) and exercise intensity [44,45]. In addition, intramuscular carnosine levels are affected by muscle fiber type composition (types I and II) and training status [23]. Therefore, the effect of carnosine/anserine supplementation may differ between athletes and nonathletes (sedentary or active men), and further studies are warranted.
In summary, these results found that while hemolysis and increased hepcidin concentration occurred after an 80 min-running session, long-term carnosine/anserine supplementation does not affect iron regulation post-exercise in active males.
Acknowledgements
We thank all participants in this study for completing the long-term carnosine/anserine supplementation and the four exercise sessions. We also thank the laboratory members and NH Foods Ltd. for their technical support. This study was financially supported by NH Foods Ltd.
This study was financially supported by NH Foods Ltd. An author (M.S.), is affiliated with NH Foods Ltd. R&D Center, involved in the development of powdery imidazole dipeptide (carnosine/anserine) supplements. M.S. contributed to this study (e.g., preparing the carnosine/anserine and placebo supplement, development of methodology), but was not involved in data collection, manuscript preparation, discussion, and drawing the conclusion, in terms of conflict of interest.