Prenatal physical activity and the gut microbiota of pregnant women: results from a preliminary investigation
Article information
Abstract
[Purpose]
To determine whether physical activity (PA), specifically meeting the recommended 150 minutes of moderate-intensity PA per week, is associated with gut microbiota composition in pregnant women.
[Methods]
In an ongoing birth cohort study, questions from the Behavioral Risk Factor Surveillance System, which provides data on PA variables, were used to determine whether pregnant women met or exceeded the PA recommendations. To profile the composition of gut bacterial microbiota, 16S rRNA sequencing was performed on stool samples obtained from pregnant women. Differences in alpha diversity metrics (richness, Pielou’s evenness, and Shannon’s diversity) according to PA were determined using linear regression, whereas beta diversity relationships (Canberra and Bray-Curtis) were assessed using Permutational multivariate analysis of variance (PERMANOVA). Differences in relative taxon abundance were determined using DESeq2.
[Results]
The complete analytical sample included 23 women that were evaluated for both PA and 16S rRNA sequencing data (median age [Q1; Q3] = 30.5 [26.6; 34.0] years; 17.4% Black), and 11 (47.8%) met or exceeded the PA recommendations. Meeting or exceeding the PA recommendations during pregnancy was not associated with gut microbiota richness, evenness, or diversity, but it was related to distinct bacterial composition using both Canberra (p = 0.005) and Bray-Curtis (p = 0.022) distances. Significantly lower abundances of Bacteroidales, Bifidobacteriaceae, Lactobacillaceae, and Streptococcaceae were observed in women who met or exceeded the PA recommendations (all false discovery rates adjusted, p < 0.02).
[Conclusion]
Pregnant women who met or exceeded the PA recommendations showed altered gut microbiota composition. This study forms the basis for future studies on the impact of PA on gut microbiota during pregnancy.
INTRODUCTION
In 2018, the US Department of Health and Human Services released the second edition of the Physical Activity Guidelines for Americans, noting the importance of pregnant women completing at least 150 minutes of moderate-intensity physical activity (PA) throughout the week [1]. During pregnancy, PA is associated with improved maternal–child health outcomes, including a reduced risk of excessive weight gain, gestational diabetes mellitus (GDM) [1-3], pre-eclampsia, cesarean section (C-section) delivery1, and preterm birth (PTB) [2].
Thus, PA has emerged as a potential modulator of the human gut microbiome [4-8]. In animal models, PA independently altered the composition and functional capacity of the gut microbiota [9-12], and increased the relative abundance of butyrate-producing taxa [13]. Although limited by their cross-sectional designs, several studies have shown that PA influences the gut microbiota in non-pregnant humans [14-16]. For example, increased levels of Faecalibacterium prausnitzii, Roseburia hominis, and Akkermansia muciniphila have been observed in non-pregnant women performing at least 3 hours of PA per week [14]. Moreover, longitudinal intervention studies ranging from 6 to 8 weeks suggest that PA has independent effects on the gut microbiota [17,18]. However, little is known about the relationship between PA and the gut microbiota during pregnancy, despite evidence that the gut microbiota plays a role in prenatal health [19-25]. Studies on PA and microbiota during pregnancy have primarily been conducted in animal models. Rats with greater PA levels during pregnancy had a decrease in their microbiota α-diversity as microbes beneficial to gut health were transferred to their offspring [26,27]. Zhou et al. [27] have demonstrated that PA consistently contributed to an enriched genus Odoribacter in pregnant rats and their offspring, thereby improving metabolic health. However, no study has investigated the effects of PA on the gut microbiome of pregnant women.
Thus, in this study, we aimed to explore the effects of PA during pregnancy on the maternal prenatal gut microbiota composition in a subgroup of participants from the Archive for Research on Child Health (ARCH) pregnancy cohort, ARCHGUT.
METHODS
Study population
The details of the ARCH cohort [28] and ARCHGUT subgroup [29] have already been published. In brief, ARCHGUT was an optional extension of ARCH and enrolled English-speaking pregnant women ≥ 18 years old between 2015 and 2017. The ARCH study ended in 2018. ARCHGUT enrolled 29 participants receiving prenatal care in Michigan’s Lansing and Traverse City areas. Written informed consent was obtained from all the participants. This study was approved by the Michigan State University Biomedical and Health Institutional Review Board (#14-170M).
PA assessment during pregnancy
PA was assessed prenatally using the Behavioral Risk Factor Surveillance System questions [30]. The primary explanatory variable was defined as whether the participant was meeting or exceeding the PA recommendations for pregnant women (i.e., ≥ 150 minutes of moderate (or more intense) PA/week) [31].
Additional covariates
Indicators of prenatal diet (daily servings of fruits and vegetables (excluding French fries), dairy, and added sugar) were assessed using a Five-Factor Screener based on the 2005 National Health Interview Survey Cancer Control Supplement (NHIS) Questionnaire [32]. Other self-reported covariates during prenatal visits included ethnicity, education, household income, marital status, parity, and pre-pregnancy body mass index (BMI). Maternal age at PA questionnaire (years), gestational age at PA questionnaire (weeks), and season at PA questionnaire were also included.
Stool specimen collection and microbiota profiling
The participants provided stool samples during the third trimester using ParaPak Clean Vials (Meridian Biosciences, Cincinnati, OH, USA) [33] for sample collection. No instructions on the timing of stool collection or the requirements for defecation relative to eating and drinking were given to the participants. The 16S rRNA sequencing was used to profile the bacterial gut microbiota, and DNA extraction and amplification were performed as previously described [34]. The rarefied OTU table was used to calculate alpha (richness, Pielou’s evenness, and Shannon’s diversity) and beta (Canberra and Bray-Curtis) diversity metrics.
Statistical analysis
The statistical significance level was set at 0.05. All analyses were performed using R (R Foundation for Statistical Computing, Vienna, Austria) version 4.2.0. Maternal factors associated with meeting or exceeding the PA recommendations were assessed using the Kruskal–Wallis test for continuous covariates and the Chi-square test for categorical covariates. Fisher’s exact test was used when expected frequencies were less than five in some cells. Differences in alpha diversity metrics were tested using linear regression, whereas beta diversity was tested using Permutational multivariate analysis of variance (PERMANOVA) [35]. Taxonomic testing was performed using DESeq2 [36] on the unrarefied OTU table, with Benjamini and Hochberg’s [37] false discovery rate (FDR)-adjusted p-value < 0.05, which was considered significant.
RESULTS
Complete data were available for 23 women (17.4% Black). A total of 11 (47.8%) met or exceeded the PA recommendations. The detailed maternal characteristics based on whether they met or exceeded the PA recommendations are shown in Table 1. The women who met the PA recommendations were older (p = 0.002), more likely to be white (p = 0.014), and more likely to be married (p = 0.037). However, no differences were found by pre-pregnancy BMI (p = 0.718) or any dietary indicators (all p ≥ 0.45).
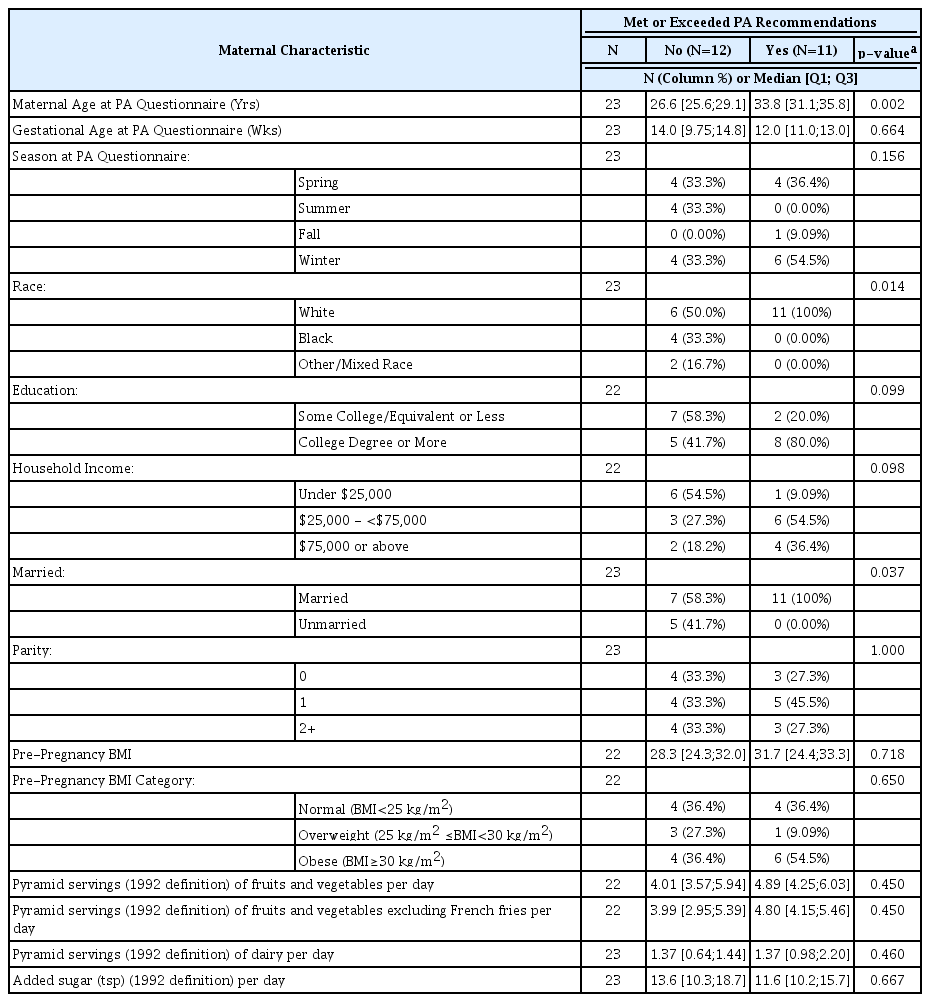
Association between maternal characteristics and meeting physical activity (PA) recommendations (N=23).
Meeting the PA recommendations during pregnancy was not associated with gut richness (p = 0.33), evenness (p = 0.57), or diversity (p = 0.23) (Figure 1), but was associated with distinct bacterial composition using both Canberra (p = 0.005) and Bray-Curtis (p = 0.022) distances (Figure 2). Meeting or exceeding PA recommendations explained 5.7% and 7.9% of the variability in the stool samples for Canberra and Bray-Curtis distances, respectively. Moreover, clustering was visually present, most prominently using the Canberra distance.
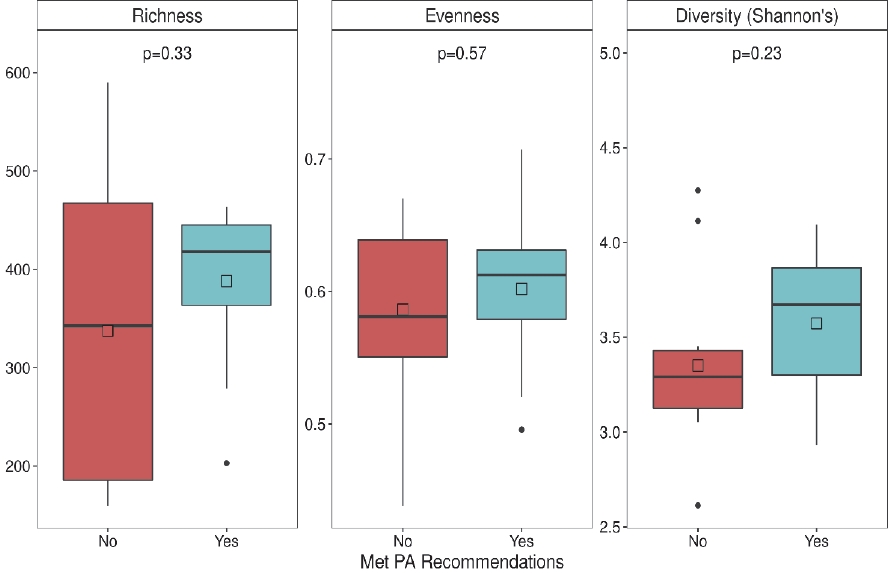
Stool alpha diversity metrics of women who did or did not meet physical activity (PA) recommendations. Two-sample t-test p-values are shown.
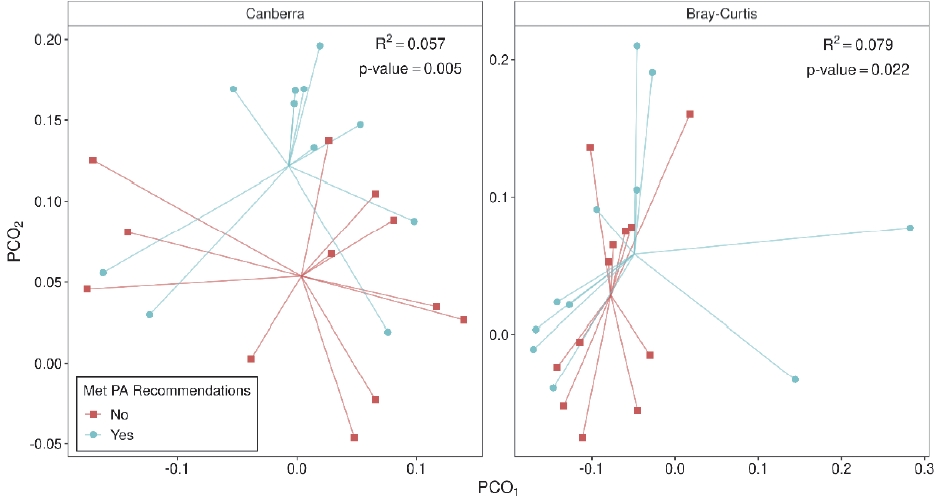
Gastrointestinal microbiota composition based on the (A) Canberra metric and (B) Bray-Curtis metric in women who did or did not meet physical activity (PA) recommendations. PERMANOVA R2 and p-values are shown.
We formally tested for differences in family level abundance within these groups (Figure 3). Four families were differentially abundant, all of which had a significantly lower abundance in women who met or exceeded the PA recommendations. These families included Bacteroidales (log2FC = -4.98; pFDR = 0.002), Bifidobacteriaceae (log2FC = -2.10; pFDR = 0.002), Lactobacillaceae (log2FC = -4.16; pFDR = 0.003), and Streptococcaceae (log2FC = -2.09; pFDR = 0.020).
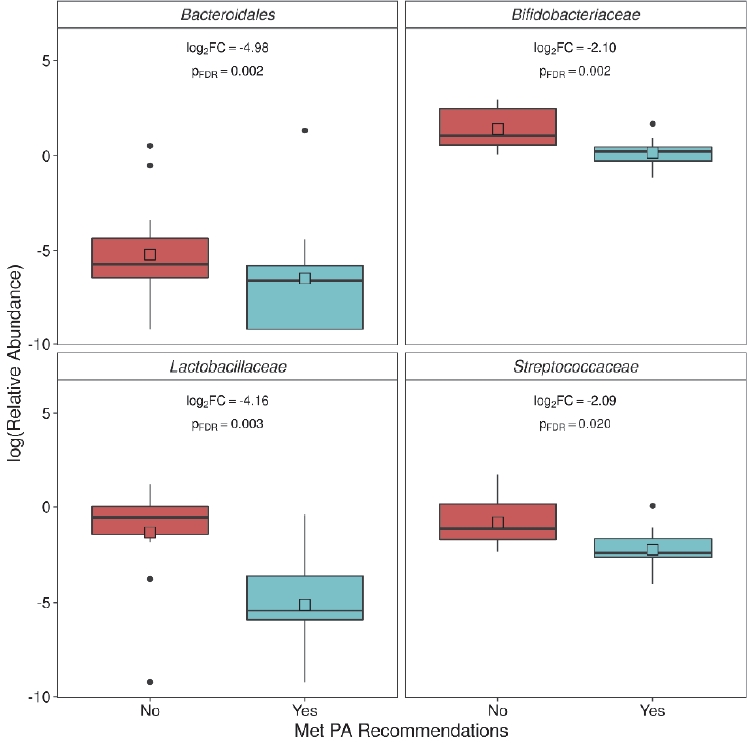
Families significantly negatively associated with meeting physical activity (PA) recommendations. Log2 fold change (FC) estimates and false discovery rate (FDR) corrected p-values calculated using DESeq2 (pFDR<0.05 considered significant).
When specific OTUs were tested for differential abundance (Supplementary Material Table 1), 21 units were significant. The results were generally consistent with family level tests, such as one Bifidobacteriaceae OTU and two Lactobacillaceae OTUs, with significantly lower abundances in women who met or exceeded PA recommendations. However, four Lachnospiraceae OTUs were also significantly more abundant in women who met or exceeded the PA recommendations.
DISCUSSION
In this preliminary analysis of the effect of PA on the gut microbiota of pregnant women, we found that the composition of the gut microbiota differed between women that had met or exceeded the PA recommendations and those that had not. Previous research in a nationally representative sample suggests that the prevalence of pregnant US citizens meeting the guidelines for at least 150 minutes of moderate-intensity PA throughout the week is approximately 39% [38], which is lower than the 47% observed in our cohort study. PA is known to affect the metabolic [39-41], gene regulation [42], and signal transduction [43] biological pathways. However, it cannot explain the total positive impact of PA on health. Understanding the impact of this important health-promoting behavior on gut bacterial communities may elucidate the mechanistic pathways through which these commensal microbes alter their biology and contribute to positive health outcomes.
Several gut microbiota constituents were altered in women who met or exceeded the PA recommendations. An association between changes in the concentration of some of these organisms and pregnancy complications has been reported, which are also associated with PA. For example, a higher relative abundance of Bacteroidales has been reported in pregnant women with pre-eclampsia [44] and overweight pregnant women [45]. We found that women meeting or exceeding the PA recommendations had a lower abundance of Bacteroidales, and studies have reported that PA is associated with a decreased risk of pre-eclampsia [1,46] and a reduced risk of excessive weight gain [1-3]. In an analysis of stool samples from 19 women who gave birth prematurely and 102 who had a full-term birth, species of the Bifidobacterium genus were reduced in women who delivered prematurely [47]. Similarly, pregnant women with GDM have reduced numbers of Bifidobacterium species in the gut [48]. Further, we found that women meeting or exceeding the PA recommendations had a lower abundance of Bifidobacteriaceae, however, PA is associated with decreased risk of PTB [2] and GDM [1-3]. These conflicting findings emphasize the need for future research to better understand the impact of PA on gut bacterial communities. Moreover, maternal PA and the associated alterations in the gut microbiota have been explored in a limited manner in humans, with no known published journal articles. This highlights the paucity of research in this area. In the current study, we utilized the 16S rRNA sequencing technology to evaluate the gut microbiota composition, which does not provide functional information. Future studies utilizing technologies such as metagenomics, metabolomics, and/or metatranscriptomics [49] to quantify the potential functional differences associated with exercise are needed to better understand the potential microbiological pathways between PA and maternal and delivery health outcomes.
The current study has some limitations. One primary limitation is that this study was not designed to focus on PA assessments. Future investigations are needed to accurately document the daily course of pregnancy-related changes in PA and sedentary behavior [50]. Such studies must be designed specifically to test the impact of PA on the pregnant gut microbiome and should consider using a specific maternal PA self-reported assessment, such as the Pregnancy Physical Activity Questionnaire (PPAQ) [51]. As underestimation and overestimation of self-reported PPAQ measures have been documented in the literature [52], investigators should also consider utilizing objective measures of maternal PA. An example of an objective PA measurement tool is an activity tracking device (ATD). ATDs appear to be more accurate than self-reported measures in pregnant populations [52-54]. Moreover, in pregnant women, ATDs are both valid [54] and feasible [52,54]. Furthermore, in our study, the sample primarily consisted of white women with PA recorded at a single time point during pregnancy using a subjective measure. Information regarding the time-of-day stool collection or the time since the most recent eating/drinking occasion was not collected. Future studies should consider asking participants to report defecation in relation to eating and drinking. A recent study in animal models using a diet and exercise intervention suggested that changes in maternal gut microbiota in response to an exercise intervention are diet dependent [55]. This suggests the need for a detailed dietary history, which was not obtained in the current study. Therefore, as diet is a key modifiable factor that influences the composition of the gut microbiota during pregnancy, future studies should consider gathering in-depth dietary intake details using 24-hour dietary recall and/or food frequency questionnaires. Although the small sample size of the current study has its limits, it provides preliminary results that form a basis for hypotheses that can be tested in future studies. Understanding the effect of PA on the gut microbiota during pregnancy requires additional studies to fully understand the impact of PA on positive health outcomes.
Supplementary Material
OTUs significantly associated with meeting physical activity (PA) recommendations in ARCH women.
Acknowledgements
This research was supported by the Office of the Director at the National Institutes of Health under award numbers UG3OD023285 and UH3OD023285 and by the Michigan State University Center for Research in Autism, Intellectual, and Neurodevelopmental Disabilities (C-RAIND). This research could not have been conducted without the collaboration of the Sparrow Hospital, MSU Faculty Clinic, and MSU Residency Clinic in Lansing, MI.
We thank the participants in the CHARM study.