Comparison of three type of muscle glycogen loading interventions using a very-high-carbohydrate diet in an elite male racewalker: a case report
Article information
Abstract
[Purpose]
Muscle glycogen storage before a race is necessary for endurance athletes to achieve the best performance. Generally, the recommended carbohydrate intake for preparation over 90 min of the race is 10–12 g·kg-1·day-1. However, it remains unclear whether an elite athlete with an already high-carbohydrate diet can further increase muscle glycogen through a very-high-carbohydrate intake. Therefore, we compared the effects of three types of glycogen loading in a 28-year-old male athlete who belongs to the top 50 racewalkers in the world, consuming a daily energy intake of 4507 kcal and a carbohydrate intake of 12.7 g·kg-1·day-1.
[Methods]
The racewalker consumed very-high-carbohydrate diets three times for 2 days each, 13.7 g·kg-1·day-1 for trial 1, 13.9 g·kg-1·day-1 for trial 2, and 15.9 g·kg-1·day-1 for trial 3. Muscle glycogen concentrations in the anterior (vastus lateralis and vastus intermedius) and posterior thighs (semimembranosus, semitendinosus, and biceps femoris) were measured using carbon-13 magnetic resonance spectroscopy.
[Results]
Muscle glycogen concentrations in both the anterior and posterior thighs increased in all trials, particularly in trial 3. Body mass also increased by 1.5 kg in trials 1 and 2 and by 1.8 kg in trial 3 before and after the trials. The participant felt satiated throughout the day and experienced stomach discomfort during trial 3.
[Conclusion]
We found that a 2-day very-high-carbohydrate diet and tapering of training could further increase the muscle glycogen concentration in athletes. However, we speculated that 15.9 g·kg-1·day-1 carbohydrate intake was unrealistic for the actual pre-race diet.
INTRODUCTION
Racewalk events were held at 20 km and 50 km in the Olympics and world championships until 2021 and have been changed to 20 km and 35 km since 2022. As of March 2022, the men’s world record for the 20-km racewalk was held by Suzuki Yusuke, who completed the race at 1:16:36, and that for 50 km was held by Diniz Yohann, who completed it at 3:32:33 [1]. The average walking speeds for these races were calculated from these records as 15.7 and 14.3 km·h-1, respectively, and their metabolic equivalents (METs) were expected to be approximately 14 and 12 METs, respectively [2,3]. These records indicate that racewalking is a high-intensity endurance exercise and a tough sport.
High-intensity endurance exercise relies on carbohydrates as an energy source [4]. To win a race, athletes are trained to preserve their muscle glycogen as much as possible until the end of the race so that they can make a last spurt. For these endurance athletes, especially 50-km racewalkers, unique nutritional strategies such as carbohydrate loading have been proposed to prepare them for races [5]. The general recommended amount of carbohydrate for the preparation over 90 min of the race is 10–12 g·kg-1·day-1 for 36–48 h [5,6]. Here, we report a rare case of an athlete whose habitual carbohydrate intake was over 12 g·kg-1·day-1. Habitual high carbohydrate diets increase carbohydrate oxidation and muscle glycogenolysis [7]. Furthermore, stored glycogen is limited to 300–700 g in the muscles and 100–120 g in the liver of a reference male weighing 75 kg [4]. Thus, it was unclear whether increasing the carbohydrate intake beyond the recommended level would further increase muscle glycogen concentration in elite racewalkers. Excessive carbohydrate intake can potentially further increase muscle glycogen levels and body fat.
Herein, we report a case study of carbohydrate loading with a very-high-carbohydrate diet in an elite racewalker who habitually consumed a high-carbohydrate diet to recover from extreme daily training. The racewalker desired to increase his muscle glycogen concentration further to win the race. Therefore, we measured the muscle glycogen concentration (at two sites on his right leg), body mass, and condition, and evaluated the very-high-carbohydrate diet intake to improve his performance.
METHODS
Characteristics of the athlete
The athlete was a 28-year-old male who was a 50-km racewalker and belonged to the top 50 in the world, the top 20 in Asia, and the top eight in Japan. His initial height, body mass, and body fat percentage were 172.7 cm, 62.6 kg, and 11.9%, respectively. The athlete provided written informed consent to participate in the study and shared his data in the paper. This study was approved by the Ethics Committee of the Japan Institute of Sports Sciences (approval no. 030, 2020).
Nutritional intervention
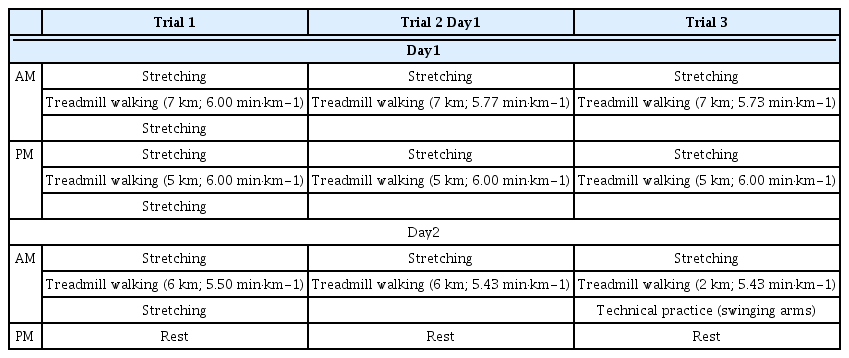
The nutritional intervention schedule is shown in Figure 1. Carbohydrate loading trials were conducted three times, with a 3-week interval between trials 1 and 2, and a 6-week interval between trials 2 and 3. The racewalker consumed only the prescribed diet based on the 3-day food record (baseline). Although the energy and macronutrient content during trials 1 and 2 were similar, the energy and macronutrient content in breakfast in trial 2 was less to reduce gastrointestinal discomfort after breakfast (before training), and in the snack was more substantial than that in trial 1 (Tables 1 and 2). The nutritional intervention period was between breakfast on days 1 and 3 (2-day trial; Figure 1). Registered dietitians specific to clinical sports nutrition prepared a menu of very-high-carbohydrate diets based on energy and macronutrient intake at baseline.
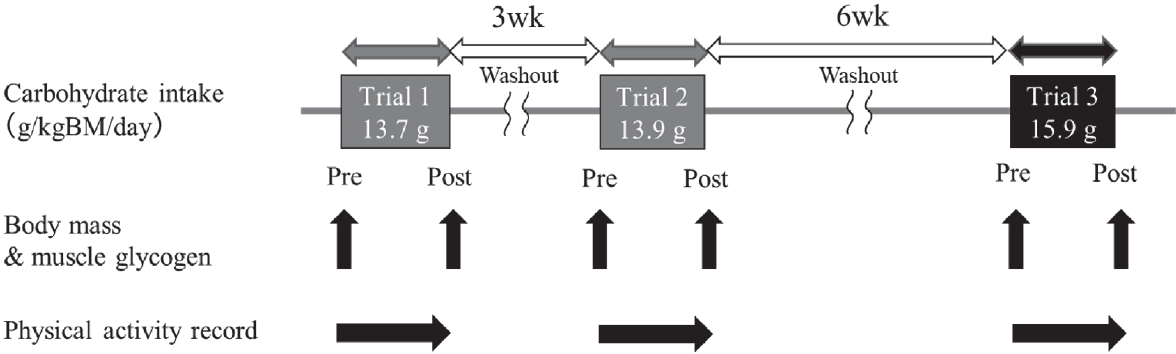
Nutritional intervention schedule.
Body mass and muscle glycogen were measured pre and post of each trial. Physical activity was recorded during each trial.
Assessment of energy and macronutrient intakes
Habitual energy and macronutrient intakes were determined using a self-administered food record with photographs taken 3 days before the 2 weeks of nutritional intervention, including two training days and one off-training day as baseline. Registered dietitians calculated these intakes using the original software, according to the Standard Tables of Food Composition in Japan 2020 (Mellon II; SOFTOM Co., Ltd, Tokyo, Japan).
Measurement of physical activity
Physical activity throughout the trials and baseline period was recorded using a triaxial accelerometer (Active Style Pro HJA-750C; Omron Healthcare Co., Ltd, Kyoto, Japan). The athlete was asked to wear the device on his waist during all activities, except bathing and dressing. There was no training in water. The physical activity intensity thresholds were defined as follows: sedentary, ≤1.5 metabolic equivalents (METs); light physical activity (LPA), 1.5–2.9 METs; moderate physical activity (MPA), 3.0–5.9 METs; and vigorous physical activity (VPA), ≥6.0 METs [8]. We instructed the athlete to perform similar physical activities and training as much as possible during all trials. The resting metabolic rate was estimated using the estimation equation [9], and the activity energy expenditure was calculated by multiplying the resting metabolic rate per minute by the METs of each activity. Daily total energy expenditure was calculated by adding resting metabolic rate and activity energy expenditure with dietary induced thermogenesis as 10% [10].
Measurement of body mass, impedance, and muscle glycogen concentration
Body mass, impedance, and muscle glycogen concentration were measured after breakfast on days 1 (pre) and 3 (post) (Figure 1). The athlete was instructed to consume breakfast for at least 2 hours before these measurements and to ingest water as desired during all trials. Furthermore, the athlete was allowed to drink water for up to 1 h before the assessment. Body mass and impedance values were measured using a bioelectrical impedance analysis device (InBody 730; InBody Co., Ltd, Seoul, South Korea) with a scale nearest 100 g. The athlete cleaned the soles of feet and palms with electrolytic tissue and stood barefoot on a scale wearing light sportswear. Body mass and impedance were measured according to the manufacturer’s instructions. We analyzed the Z values detected in each body part (right arm, left arm, trunk, right leg, and left leg) at a frequency of 50 kHz.
Muscle glycogen concentrations were measured with carbon-13 magnetic resonance spectroscopy using a 3-T superconducting magnetic resonance (MR) scanner (Magnetom Verio, Siemens Co., Ltd, Erlangen, Germany) for the anterior thigh (vastus lateralis and vastus intermedius), which constitutes half of the thigh length, and a similar MR scanner (Magnetom Skyra, Siemens Co., Ltd) for the posterior thigh (semimembranosus, semitendinosus, and biceps femoris), which spans proximally a third of the thigh length. The athlete lay on his back for measurement and fixed his right leg on a 13C-1H double-tuned surface coil. The 13C-glycogen signal was obtained as the sum of 4500 scans with a repetition time of 200 ms. Muscle glycogen concentration was calculated using a glycogen cylindrical phantom of known concentration (120 mM glycogen from oysters and 50 mM KCL)11. The coefficient of variation of repeated measurements of muscle glycogen concentration using this method with repositioning and reshimming was 3.5% in individuals in the previous study [11].
RESULTS
The initial body mass was 63.0, 62.4, and 62.8 kg at trials 1, 2, and 3, respectively. Body mass increased after the 2-day glycogen loading period in all trials, and the increase in trial 3 was greater than that in the other trials (Table 3). Total body water increased in all trials. The Z values in all body regions decreased after glycogen loading, and the decrease in trials 2 and 3 was greater than that in trial 1. The initial muscle glycogen concentrations in the anterior thigh were 58.6, 59.4, and 101.4 mM in trials 1, 2, and 3, respectively, and those in the posterior thigh were 87.9, 108.4, and 81.9 mM in trials 1, 2, and 3, respectively (Figure 2). The muscle glycogen concentration in both the anterior (17.6 mM, 30% in trial 1; 42.6 mM, 72% in trial 2; 18.1 mM, 18% in trial 3) and posterior thighs (29.5 mM, 34% in trial 1; 40.2 mM, 37% in trial 2; 99.1 mM, 121% in trial 3) was increased in all glycogen loading trials. The sum of the increases in muscle glycogen concentrations of the anterior and posterior thighs was 47.1 mM in trial 1, 82.8 mM in trial 2, and 117.2 mM in trial 3.

Change in muscle glycogen concentration at the anterior and posterior right thigh.
The data of the anterior thigh in (a), and the posterior right thigh in (b). White circle and dashed line express trial 1, gray circle and gray solid line express trial 2, and black triangle and black solid line express trial 3. Change in muscle glycogen concentration in (c). Relative change in muscle glycogen between pre and post in (d). White bar expresses anterior thigh and black bar expresses posterior thigh.
The training menu for three-day baseline period consisted of the following components: AM, stretching, warming-up (12.50 min·km-1 × 1.2 km), interval training (2 km × 3 times + 200 m × 5 times), cooling-down; PM, stretching, weight training, racewalking (5.50 min·km-1 × 14 km) during day 1; AM, Rest; PM, stretching, racewalking (6.00 min·km-1 × 10 km), technical practice (drill); Night, technical practice (swinging arms), bodyweight workout during day 2; AM, stretching, racewalking (6.00 min·km-1 × 10 km), technical practice (drill); PM, long distance racewalking (4.96 min·km-1 × 25 km) during day 3. The training menus used during the three trials are listed in Table 4. Although the training menus were similar among the three trials, the treadmill walking speed in the morning during trials 2 and 3 was faster than that during trial 1. In all the trials, the daily VPA time was lower than that at baseline, whereas the sedentary time increased (Figure 3). Among the three trials, the sedentary time in trial 1 was slightly longer than in the other two trials. The total energy expenditure during all trials (trial 1, 2846 kcal·d-1; trial 2, 3036 kcal·d-1; trial 3, 2823 kcal·d-1) was lower than baseline (4435 kcal·d-1). The activity energy expenditure among three trials (trial 1, 1096 kcal·d-1; trial 2, 1269 kcal·d-1; trial 3, 1074 kcal·d-1) were lower than baseline (2541 kcal·d-1), and it in trial 2 was highest among three trials. The maximum activity energy expenditure per minute during the three trials (trial 1, 11.2 kcal·min-1; trial 2, 17.4 kcal·min-1; trial 3, 12.9 kcal·min-1, Figure 5) was also lower than baseline (19.5 kcal·min-1, Figure 4). In addition, the maximum activity energy expenditure per minute in trial 2 was the highest among the three trials.

The time of each physical activity in metabolic equivalents (METs) across the three trials and baseline.
Gray bar, slanted line, white bar, and black bar expressed as sedentary (≤1.5 METs), light physical activity (1.5–2.9 METs), moderate physical activity (3.0–5.9), and vigorous physical activity (>6.0 METs), respectively.

Change in activity energy expenditure per minute through the baseline recording period.
The dots represent energy expenditure per minute.
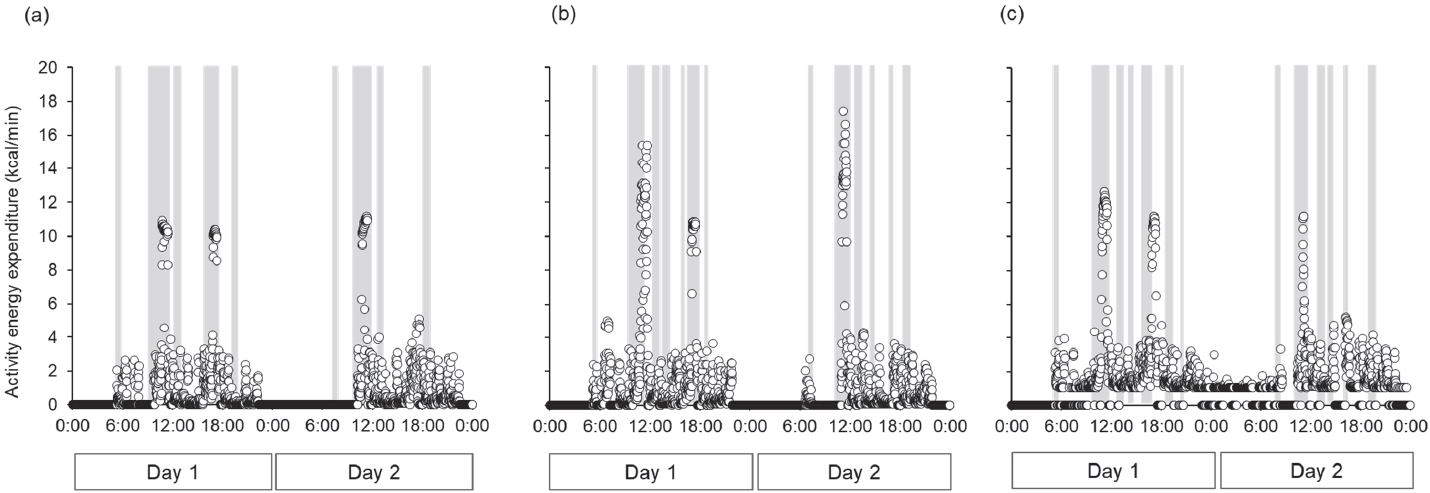
Change in activity energy expenditure per minute during the three trials.
(a) trial 1, (b) trial 2, (c) trial 3. The dots represent the energy expenditure per minute. Gray bar expressed as meal timing (breakfast, lunch, dinner, refuel during the training, and snacks).
We also obtained the following subjective findings through interviews after the athlete underwent the three trials (Table 5). In trial 1, the participant felt satiated because the breakfast was large. However, in trial 2, the feeling of satiety at breakfast disappeared. In trial 3, because the amount of food for lunch and dinner was increased, he felt satiated and experienced stomach discomfort throughout the day; he considered that 15.9 g·kg-1·day-1 of carbohydrate intake was unrealistic for the actual race. Finally, he decided on a meal containing 14.5 g·kg-1·day-1 of carbohydrate for the actual race after completing this experiment.
DISCUSSIONS
In this case report, we compared three strategies of muscle glycogen loading in an elite athlete who had already been consuming a carbohydrate diet higher than recommended (12.7 g·kg-1·day-1). To our knowledge, this is the first case report to show that a very-high-carbohydrate diet (15.9 g·kg-1·day-1) in combination with tapering of training was beneficial for increasing muscle glycogen concentration. However, we considered that the very-high-carbohydrate diet in trial 3 was too high to be used in an actual race.
The differences in the changes in muscle glycogen concentrations in the anterior and posterior thigh muscles during the three trials were not the same. However, the change in the sum of muscle glycogen concentration and body mass was greater in trial 3 than in the other trials. Furthermore, the total body water increased and the impedance values in all body regions decreased with changes in muscle glycogen concentration. The impedance at a frequency of 50 kHz represents the total body water resistivity [12]. Generally, increased muscle glycogen concentration is associated with increased body mass as each gram of accumulated muscle glycogen is bound with 3–5 g of water [4,13,14]. Although we could not estimate the total muscle glycogen accumulation, as we measured muscle glycogen from only two sides, we considered that the increase in body mass, total body water, and the decrease in the impedance values were related to the muscle glycogen accumulation. These findings suggest that a very-high-carbohydrate intake exceeding the recommended values may be useful for pre-race glycogen accumulation in racewalkers who routinely consume a high-carbohydrate diet. However, athletes and coaches should be aware of body mass gain due to glycogen loading. In this study, the athlete showed a weight gain of 2.4%–2.9%, which is more than that previously reported [4,14].
To maximize muscle glycogen concentration, athletes and coaches must consider training volume and intensity prior to the race. In this study, the training volume and intensity decreased during the three trials to imitate tapering during the pre-race period (Figure 3, 4, and 5). This is believed to have contributed to an increase in the muscle glycogen concentration. A previous study showed that a high-carbohydrate (10 g·kg-1·day-1) and high-energy diet in endurance athletes who normally consume 5.8 g·kg-1·day-1 of carbohydrates induced a 90% increase in muscle glycogen concentration for 24 h without any exercise [15]. Another study reported that a high-carbohydrate diet (70% of energy) after a 3-day normal-carbohydrate intake (50% of energy) in conjunction with depletion-tapering exercise (decrease work time) also induced a 52% increase in muscle glycogen concentration [16]. These findings are consistent with ours in that despite engaging only in light exercise (middle-vigorous exercise time was decreased by 56 % in trial 1, 53% in trial 2, and 58% in trial 3), the athlete succeeded in increasing muscle glycogen concentration within 2 days without glycogen-depleting exercise.
The amounts of energy and carbohydrates in trials 1 and 2 were similar. Nevertheless, the increase in muscle glycogen in trial 2 was greater than that in trial 1. One of the reasons for this phenomenon might be the amount of carbohydrate per meal or snack, because intestinal carbohydrate digestion and absorption rates are limited [17]. Our test meals and snacks consisted of whole food and sports foods, such as sports drinks and gels, in every trial. The ingestion of carbohydrates with other nutrients (e.g., protein and fat) attenuates gastric emptying and intestinal absorption [18]. Therefore, the muscle glycogen recovery rate was slower in trial 1, in which more energy and macronutrients were consumed at breakfast than in trial 2, in which more sugar was ingested as snacks. Another reason may be that the slightly higher exercise intensity in trial 2 (Table 4, Figure 5) may have increased muscle glycogen consumption and recovery. Glycogen uptake into the muscles increases after high-intensity exercise, which depletes muscle glycogen [19]; however, we did not measure the amount of glycogen consumed after exercise. Our data showed that body mass and total body water did not differ between trials 1 and 2. Furthermore, the decrease in leg impedance in trial 2 was greater than that in trial 1. This was consistent with changes in muscle glycogen concentration. However, the decline in the impedance values of the arms and trunk in trial 2 was less than that in trial 1. These data suggest that the distribution of digested carbohydrates in the body may have differed depending on the muscle utilized during exercise and exercise intensity between trials 1 and 2.
Muscle glycogen utilization is affected by the movements performed in sports and the muscle groups involved [20,21]. In our case, the muscle glycogen concentration in the posterior thigh increased dramatically (Figure 2). In racewalking, athletes are required to straighten their legs from the moment of first contact with the ground until they reach a vertical upright position and are not allowed to take their feet off the ground simultaneously [22]. Therefore, world-class race walkers produce energy from their hip flexors during swinging to ensure this characteristic walking posture [23]. These unique movements may induce greater synthesis and use of glycogen in the posterior thigh muscles than in the anterior thigh muscles. Furthermore, differences in muscle fiber types between the anterior and posterior thigh may affect muscle glycogen accumulation. Future studies should investigate the relationship between the distribution of muscle fiber types and glycogen recovery in these muscles.
A limitation of the present study is that the effects of a very-high-carbohydrate diet could only be evaluated based on changes in muscle glycogen. Therefore, future studies should assess the relationship between exercise performance and physiological parameters such as muscle and liver glycogen, blood glucose, insulin, glucagon, and lactate levels to better understand their effects. Moreover, we were not able to completely control the food intake and training volume before each trial, and the difference in the muscle glycogen concentration pre-among these three trials, especially trial 3, may have affected the muscle glycogen concentration. Finally, this study was conducted on only one elite athlete; therefore, further studies involving more elite racewalkers are required to validate our findings. In the future, a crossover randomized controlled trial with a large sample size and further studies on the effects of different exercise intensities during tapering on muscle glycogen accumulation are required.
In conclusion, we report a case study in which an elite race walker who regularly consumed a high-carbohydrate diet showed an increase in muscle glycogen concentration following a very-high-carbohydrate diet. Our findings from this unique case could be helpful to athletes who consume a high-carbohydrate diet and serve as a basis for future studies on this topic. However, it is important to note that our data were obtained from a single elite athlete. Additional studies are required to establish the effects of carbohydrate-loading strategies on racewalk performance and to develop stronger evidence.
Acknowledgements
The authors wish to thank the athlete who participated in this study. We are also grateful to Akiko Uchizawa for analyzing energy expenditure. This case study was supported by JSPS KAKENHI Grant-in-Aid for Scientific Research Grant No.19H04017. Furthermore, this data analysis and publication were supported by JSPS KAKENHI Grant in Aid for JSPS Fellows Grant No. 21J00492.