Feasibility of mixed herbal medicine for improving gastric function in an alcohol-induced gastritis model
Article information
Abstract
[Purpose]
Excessive exercise causes various gastric dysfunction. Gastritis is common among athletes who perform high-intensity training. Gastritis is a digestive disease involving mucosal damage caused by inflammatory reactions and oxidative stress. In this study, the effects of a complex natural extract on gastric mucosal damage and the expression of inflammatory factors were evaluated in an animal model of alcohol-induced gastritis.
[Methods]
A mixed herbal medicine (Ma-al-gan; MAG) was prepared with four natural products (Curcumae longae Rhizoma, Schisandrae chinensis Fructus, Artemisiae scopariae herba, and Gardeniae Fructus) identified by a systemic analysis using the Traditional Chinese Medicine Systems Pharmacology platform. The effects of MAG on alcohol-induced gastric damage were evaluated.
[Results]
MAG (10–100 μg/mL) significantly reduced the mRNA and protein levels of inducible nitric oxide synthase and cyclooxygenase-2 in lipopolysaccharide-stimulated RAW264.7 cells. MAG (500 mg/kg/d) effectively prevented alcohol-induced gastric mucosal injury in vivo.
[Conclusion]
MAG regulates inflammatory signals and oxidative stress and is a potential herbal medicine for gastric disorders.
INTRODUCTION
In sports medicine reports, the upper digestive symptoms are frequently reported in multi-sports athletes such as triathlon and enduro [1]. Although athletes have a higher incidence of diseases including indigestion and various diseases than the general population, athletes tend to have difficulty taking medications due to doping concerns [2,3]. In order to expect nutritional balance and therapeutic effects, it is necessary to develop customized drugs for athletes who complain of diseases. Therefore, non-clinical studies using animal models are required for the development of a gastritis treatment agent.
High-concentration alcohol treatment is widely used for the establishment of animal models of gastritis and gastric mucosal damage [4]. Reactive oxygen species (ROS) and inflammatory responses play important roles in the pathogenesis of alcoholic gastritis [5]. Inflammation plays a crucial role in defence reactions in wounded tissues to inhibit damage in surrounding cells and promote tissue regeneration; however, chronic inflammation has adverse effects on gastrointestinal tissues [6,7]. In addition, an imbalance in protective factors in the damaged gastric mucosa may lead to the excessive production of ROS and reactive nitrate species (RNS) via macrophage activation, leading to chronic inflammation and gastric cancer [8,9]. Pro-inflammatory cytokines, such as interleukin (IL)-1β, and genes encoding inflammatory factors, such as inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), are overexpressed during macrophage activation in response to destructive factors, such as endotoxins and pathogens [10,11]. Activated macrophages, which produce nitric oxide (NO) and ROS, recognize pathological cell death [12]. Antioxidant enzymes, such as superoxide dismutase-1 (SOD-1) and heme oxygenase-1 (HO-1), regulate ROS production in alcohol-induced gastric mucosal damage [13]. Therefore, the control of inflammation and oxidative stress is a therapeutic strategy for gastric disorders.
The Traditional Chinese Medicine Systems Pharmacology database and analysis platform (TCMSP) database and analysis platform contains big data on the pharmacokinetic properties of natural compounds, including drug–target networks and related drug–target–disease networks, oral bioavailability, drug similarity, and intestinal epithelial permeability [14]. Various studies have shown that natural products are safe and effective for gastrointestinal protection, providing new treatment options [15,16]. Hence, we focused on four products (ma-al-gan; MAG) for the treatment of gastritis and gastric ulcers identified by a systemic analysis of the TCMPS database. The four component herbs of MAG were Curcumae longae Rhizoma, Schisandrae chinensis Fructus, Artemisiae scopariae herba, and Gardeniae Fructus. In this study, we evaluated whether MAG can regulate lipopolysaccharide (LPS)-induced inflammation and exert protective effects against gastric mucosal damage in alcohol-induced gastritis via antioxidant and anti-inflammatory effects.
METHODS
Preparation of aqueous extracts
The plant materials, Curcumae longae Rhizoma, Schisandrae chinensis Fructus, Artemisiae scopariae herba, and Gardeniae Fructus, were purchased from Semyung University Korean Medicine Hospital (Jecheon, Korea). In MAG, 350 g of plant material (100 g of Artemisiae scopariae herba, 50 g of Gardeniae Fructus, 100 g of Schisandrae chinensis Fructus, and 100 g of Curcumae longae Rhizoma) was blended and the crude powder was precipitated with 2,000 mL of sterile deionized water at 100 °C for 2 h. The aqueous extract was concentrated and evaporated at 60 °C under vacuum conditions. The extract was dissolved in 50 mL of sterile deionized water. The aqueous extract was lyophilized by freeze-drying at -60 °C. Finally, we obtained 14.9% powder (52.37 g).
Screening of active compounds and target gene
All of the components of MAG (i.e., Curcumae longae Rhizoma, Schisandrae chinensis Fructus, Artemisiae scopariae herba, and Gardeniae Fructus) were identified based on the TCMSP. The active compounds of each natural product were screened according to pharmacokinetic parameters, including oral availability (OB) ≥ 30%, permeability (Caco-2) ≥ -0.14, and drug-like activity (DL) ≥ 0.18, in the TCMSP database. Gene targets for active compounds were found using the TCMSP database.
Gene Ontology enrichment analysis
Enriched Gene Ontology (GO) terms in the biological processes category for target genes were analysed using Cytoscape. The top 20 GO terms are listed according to the number of related molecules (P < 0.001). Active compound– target gene networks were visualized using Cytoscape 3.7.2.
Cell culture and cell viability
Mouse macrophage RAW264.7 cells were purchased from the Korean Cell Line Bank (Seoul, Korea). RAW264.7 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) with 10% foetal bovine serum (FBS) and 1% penicillin-streptomycin. Cells were seeded at 5 × 104 cells/mL in 96-well plates. The cells were treated with 0, 10, 30, or 100 μg/mL MAG for 24 h. Cells were incubated with sodium 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide for 2 h at 37 °C. Absorbance at 450 nm was measured to evaluate cell viability.
Determination of NO production
The RAW 264.7 cell medium was replaced with LPS (100 ng/mL) -containing DMEM with 10% FBS and the cells were incubated for 24 h. To measure the total concentration of NO in the medium, Griess reagent was added to 100μL of supernatant from each treatment condition and the absorbance at 520 nm was measured using a microplate reader. The NO concentration was determined using Griess reagent as described previously by Lee et al. [10].
Immunoblotting
The RAW 264.7 cells were lysed with extraction buffer (20 mM HEPES, 1% Nonidet P-40, 150 mM NaCl, 10% glycerol, 10 mM NaF, 1 mM Na3VO4, 2.5 mM 4-nitrophenylphosphate, 0.5 mM phenylmethylsulfonyl fluoride, and one tablet of complete proteinase inhibitor cocktail at pH 7.5). Cell lysates were separated using 12% polyacrylamide sodium dodecyl sulphate gels and transferred to a polyvinylidene fluoride membrane (Millipore, Bedford, MA, USA). The membrane was blocked for 1 h at 25 °C with phosphate-buffered saline and 5% bovine serum albumin. The membranes were incubated with specific antibodies, including anti-iNOS, anti-COX-2, and anti-β-actin antibodies (Abcam, Cambridge, United Kingdom), for 16 h at 4 °C. The membranes were incubated using Enhanced Chemiluminescence Kits (Amersham Pharmacia, Amersham, UK). The band intensity was analysed using the Luminescent Image Analyzer LAS-4000 (Fujifilm, Tokyo, Japan).
Reverse transcription-polymerase chain reaction (RT-PCR)
The mRNA expression levels of iNOS and COX-2 were analysed by RT-PCR as previously described [11]. Total RNA was isolated using TRIzol reagent. cDNA was synthesized using the Superscript III Reverse Transcription System, following the manufacturer’s protocol (Thermofisher, Waltham, MA, USA). PCR was performed using specific primers for mouse iNOS (forward, 5′-AGA AGG AAA TGG CTG CAG AA-3′; reverse, 5′-GCT CGG CTT CCA GTA TTG AG-3′), COX-2 (forward, 5′-CCT GTG TTC CAC CAG GAG AT-3′; reverse, 5′-CCC TGG CTA GTG CTT CAG AC-3′), and GAPDH (forward, 5′-CTC GTG GAG TCT ACT GGT GT-3′; reverse, 5′-GTC ATC ATA CTT GGC AGG TT-3′) The mRNA expression levels were quantified using an ethidium bromide-stained 1.5% agarose gel. The relative intensities of the bands were visualized and analysed using ImageJ.
Animal care and in vivo studies
Sprague–Dawley (SD) rats (male, 200 g) were purchased from Orient Bio (Gyeonggi-do, Korea). The SD rats were fed a standard feed (AIN 93G formula) and had free access to water. All rats were kept in a controlled environment (room temperature, 24 ± 2 °C; humidity, 40 ± 2%; and 12-h light-dark cycle). All experiments and animal care were conducted in conformity with the institutional guidelines of Semyung University (IACUC-20-05-01). The animals (6 weeks; n = 24) were randomized into three groups: a normal condition (UN) group, an ethanol-induced gastritis (ET) group, and a MAG treatment (ET+MAG) group. The ET group was orally administered ethanol (5 g/kg/d) and the ET+MAG group was orally administered ethanol (5 g/kg/d) and MAG (500 mg/kg/d) for 4 weeks.
Histochemistry and immunohistochemistry
After 4 weeks, all animals were euthanized and gastric tissues were dissected. The gastric tissues were fixed in 4% formalin, embedded in paraffin, and cut into 5 μm sections. The tissue fragments were cleared with xylene and serially hydrated with ethanol (70%, 80%, and 90%). Sections were stained with haematoxylin and eosin or periodic acid Schiff (PAS). Some sections were incubated overnight at 4 °C with primary antibodies, such as anti-nuclear factor (NF)-κB, anti-iNOS, anti-COX-2, anti-HO-1, and anti-SOD-1. The incubated sections were treated with biotinylated pan-specific antibodies for 1 h and incubated in ABC solution prepared according to the manufacturer’s directions (Vector laboratories, Burlingame, CA, USA). After 1 h of incubation, the sections were stained with DAB. The sample sections were examined using a light microscope (Olympus BX50, Tokyo, Japan) at 200× magnification and proteins were visualized and analysed using ImageJ.
Statistical analysis
The results are expressed as the mean ± standard deviation (SD) of at least three independent experiments (n ≥ 3). The statistical differences among experimental groups were assessed using Student’s t-tests and one-way ANOVA followed by Tukey’s range test. P < 0.05 was considered statistically significant.
RESULTS
Systematic analysis of the pharmacological activity of MAG
To explore the biological functions of MAG, including roles in the regulation of inflammation and anti-oxidant pathways, we identified active compounds in each plant from the TCMSP database and evaluated these compounds by a GO analysis using Cytoscape. First, we found disease-related protein targets using the active ingredient-related gene network for each plant from the TCMSP database. Table 1 provides an overview of the 38 active compounds of MAG. These 38 active compounds interacted with 211 proteins. Next, we analysed the biological functions of these 211 proteins. Figure 1a shows the top 20 GO terms for the 211 genes. Figure 1b shows the interaction network, visualized using Cytoscape. Two genes, Erb-B2 Receptor Tyrosine Kinase 2 (ERBB2) and IL1A, were involved in the regulation of immature T cell proliferation in the thymus. Heme Oxygenase 1 (HMOX1), Hypoxia Inducible Factor 1 Subunit Alpha (HIF1A), and Nuclear Factor and Erythroid 2 Like 2 (NFE2L2) involved were related to the regulation of transcription from TNA polymerase II promoter in response to oxidative stress.
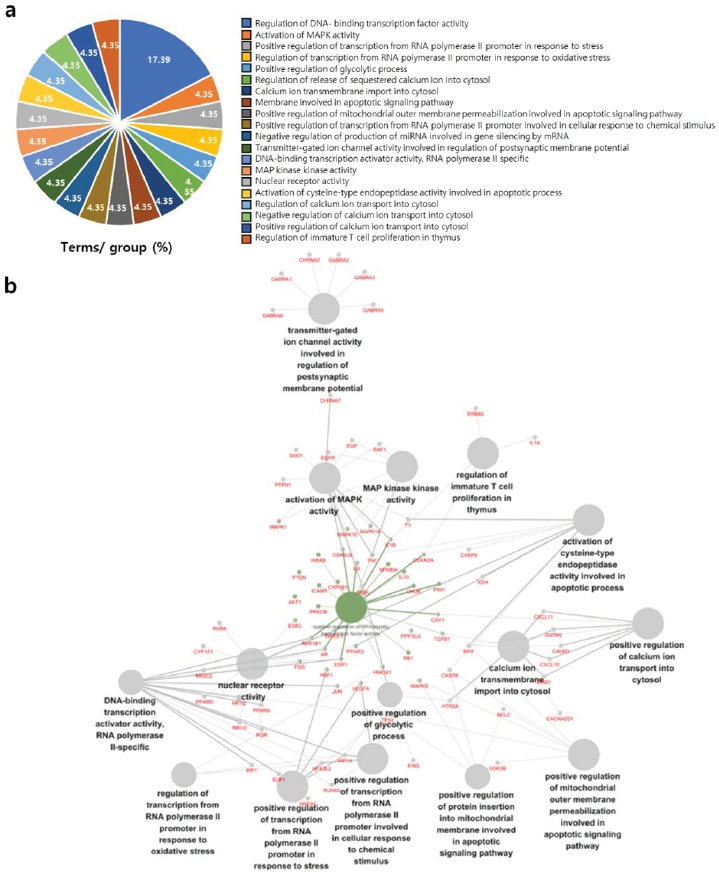
GO analysis of Ma-al-gan. The active compounds of Ma-al-gan (MAG) were analyzed using TCMSP and 211 genes related to the active compounds were obtained. Enrichment of the genes for GO biological processes was evaluated. (A) The most significant GO terms are presented; the graph was generated using Cystoscope. (B) Network analysis of selected genes.
Anti-inflammatory effect of MAG in LPS-stimulated RAW264.7 cells
We evaluated the inhibitory effect of MAG on NO production in LPS-induced RAW 264.7 cells. As shown in Figure 2, treatment with MAG (10–100 μg/mL) for 24 h did not exhibit cytotoxicity (Figure 2a). LPS treatment resulted in the production of 12.2 ± 1.2 μM NO. MAG significantly attenuated LPS-induced increases in NO production (i.e., 7.6 ± 0.1 μM, 7.9 ± 0.2 μM, and 11 ± 0.2 μM at 10, 30, and 100 μg/mL of MAG, respectively) (Figure 2b). Next, to determine the anti-inflammatory effect of MAG in LPS-induced RAW264.7 cells, the protein and RNA expression levels of inflammatory factors, such as iNOS and COX-2, were evaluated by immunoblotting and PCR, respectively. Cells were treated with or without 100 ng/mL LPS and MAG (0, 10, 30, and 100 μg/mL). In Figure 2c, the mRNA expression levels of iNOS and COX-2 in LPS-stimulated RAW264.7 cells were set to 100% and relative values for treatment groups are shown. The iNOS mRNA expression levels were reduced by 39.2 ± 2.3%, 57.5 ± 4.1%, and 52.0 ± 9.5% in cells treated with MAG (10, 30, and 100 μg/mL, respectively). The COX-2 mRNA expression levels were reduced by 58.1 ± 2.3%, 77.7 ± 2.3%, and 8.58 ± 2.6% in cells treated with MAG (10, 30, and 100 μg/mL, respectively). As shown in the Figure 2d, the iNOS protein expression levels were 39.2 ± 2.3%, 59.8 ± 4.7%, and 67.5 ± 10.2% lower in cells treated with MAG (10, 30, and 100 μg/mL, respectively) than in LPS stimulated-RAW264.7 cells. The COX-2 protein expression levels were 38.0 ± 2.3%, 46.0 ± 9.6%, and 47.6 ± 6.6% lower in cells treated with MAG (10, 30, and 100 μg/mL, respectively) than in LPS stimulated-RAW264.7 cells.

Effects of Ma-al-gan on inducible nitric oxide synthase and cyclooxygenase-2 expression in lipopolysaccharide-stimulated RAW264.7 cells. RAW 264.7 cells were treated with or without 100 ng/mL lipopolysaccharide (LPS) and 0, 10, 30, and 100 μg/mL M Ma-al-gan (MAG) for 24 h. (a) Cell viability was analysed using 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide. (b) NO production was determined using Griess reagent. (c) mRNA expression levels of iNOS and COX-2 were analysed by reverse-transcription PCR. The expression level of each mRNA in the LPS-stimulated state was set to 100%. (d) Protein expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) was evaluated by western blotting. The expression level of each protein in the LPS-stimulated state was set to 100%. Results are presented as the mean ± standard error of three independent experiments. *P < 0.05 vs. the group stimulated with LPS alone.
Gastroprotective effect of MAG against alcohol-induced gastritis in SD rat
We evaluated the gastroprotective effect of MAG against alcohol-induced gastritis. First, we performed a histological analysis using haematoxylin and eosin and PAS staining. The gastric mucosal epithelial tissue in the ET group was damaged. However, the ET+MAG group showed a similar pattern to that in the UN group (Figure 3a and b). Next, to confirm the anti-inflammatory effect of MAG on the gastric mucosal area in rats with alcohol-induced gastritis in vivo, we performed an immunohistochemistry assay with specific antibodies, including anti-NF-κB, anti-COX-2, and anti-iNOS antibodies. As shown in Figure 4, inflammatory factors (NF-κB, COX-2, and iNOS) were overexpressed in the gastric mucosal epithelial tissue in the ET group, with significantly lower levels in the ET+MAG group. Furthermore, to evaluate ROS scavenging activity, we investigated the levels of antioxidant proteins, such as HO-1 and SOD-1, in injured tissue. As shown in Figure 5, the expression levels of HO-1 and SOD-1 were higher in the ET+MAG group than in the ET group.
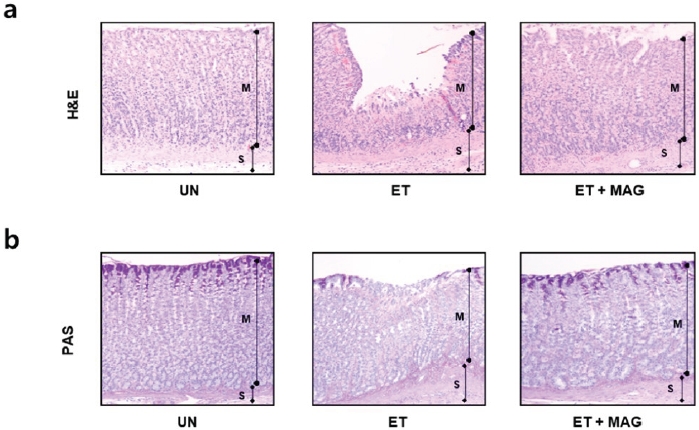
Histological changes in rats with ethanol-induced gastric injury. Rats were separated into three groups: untreated (UN), oral administration of ethanol (ET, 5 g/kg/day ethanol for 4 weeks), and co-treatment with ethanol and Ma-al-gan (MAG) (ET + MAG, 5 g/kg/ day ethanol and 5 mg/kg/day MAG for 4 weeks). (a) Gastric tissues were stained with haematoxylin and eosin (H& E). Magnification = 200×. (b) Sectioned gastric tissues were stained using periodic acid Schiff (PAS). Magnification = 200×. M, Mucosal layer; S, Submucosal layer.
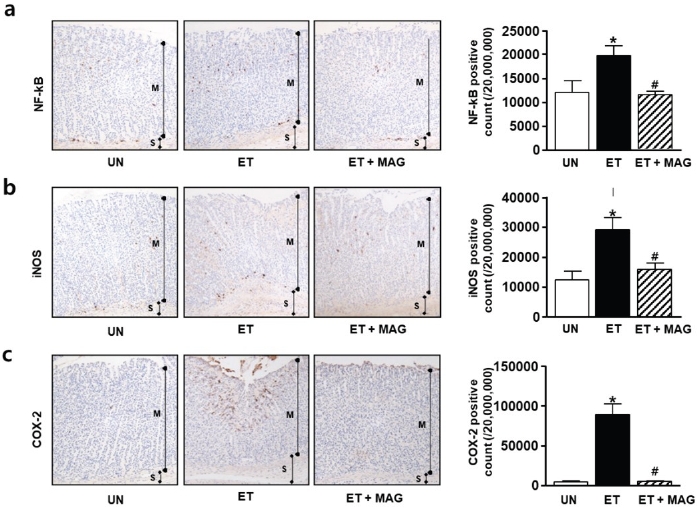
Effects of Ma-al-gan on inflammatory factors in ethanol-induced gastric injury in rats. Immunohistochemical staining of nuclear factor-κB (NF-κB) (a), inducible nitric oxide synthase (iNOS) (b), and cyclooxygenase-2 (COX-2) (c) in gastric tissues was performed with cross-sections obtained from three groups: untreated (UN), oral administration of ethanol (ET, 5 g/kg/d ethanol for 4 weeks), and co-treatment with ethanol and Ma-al-gan (MAG) (ET + MAG, 5 g/kg/ d ethanol and 5 mg/kg/d MAG for 4 weeks). Magnification = 200×. Results are presented as the mean ± standard error of three independent experiments. *P < 0.05 vs. the UN group. #P < 0.05 vs. the ET group.

Antioxidant effect of Ma-al-gan in rats with ethanol-induced gastric injury. Rats were divided into three groups: untread (UN), oral administration of ethanol (ET, 50% ethanol for 3 days), and co-treatment with ethanol and Ma-al-gan (MAG) (ET + MAG, 50% ethanol and 5 mg/kg MAG for 3 days). Representative images of the immunohistochemical detection of heme oxygenase-1 (HO-1) (a) and superoxide dismutase-1 (SOD-1) (b) expression in gastric ulcers are shown. Graphs show the protein expression levels of HO-1 and SOD-1. Results are presented as the mean ± standard error of three independent experiments. *P < 0.05 vs. the UN group. #P < 0.05 vs. the ET group.
DISCUSSION
Gastritis is defined as inflammation and ulceration of the stomach wall and has diverse causes, including overeating, the consumption of irritating foods, severe stress, smoking, Helicobacter pylori infection, nonsteroidal anti-inflammatory drugs, and excessive intake of ethanol17. In particular, the only standard treatment for erosive haemorrhagic gastritis, a disease that is accelerated by alcohol abuse, is to stop drinking alcohol [18,19]. Although gastritis caused by alcohol is mediated by increased levels of inflammatory factors, the use of anti-inflammatory drugs may increase gastric mucosal damage or cause kidney disease [20]. In addition, oxidative stress and accompanied inflammatory responses promote mucosal damage in the case of alcoholic gastritis [21]. Recent research has suggested that natural products are safe and effective gastrointestinal protectants [22,23]. We hypothesized that four natural products (MAG) selected from the TCMPS database regulate and control gastric mucosal damage in alcoholic gastritis via anti-inflammatory and antioxidant effects. We evaluated this hypothesis using an animal model of alcohol-induced gastritis and performed various assays of the therapeutic efficacy of MAG in vitro and in vivo.
The results of our in vitro and in vivo assays supported the beneficial effect of MAG on gastritis. MAG regulated NO production and inflammatory responses (e.g., iNOS and COX-2 expression) in LPS-induced RAW 264.7 cells (Figure 2). Furthermore, MAG significantly inhibited the gastro-mu-cosal damage by regulating iNOS and COX-2 expression in an alcohol-induced rat model (Figure 4). Therefore, our results demonstrate that a mixed herbal medicine, MAG, is a candidate for the treatment of gastritis.
Natural compounds with various effects, such as antioxidant, anticancer, and anti-inflammatory effects, have been identified [24]. The activity of natural extracts in vivo is typically mediated by the control of target proteins [25]. It is difficult to prove that the pharmacological efficacy of natural compounds is greater than that of Western medicines, such as small molecules and chemical drugs. However, many natural medicines have promising therapeutic effects, especially when administered over long periods [26]. Previous studies have evaluated the pharmacological effects of each of the four natural compounds in MAG. Curcumae longae Rhizoma has antioxidant, antibacterial, and anti-in-flammatory effects [27]. Gardenia fructus has liver protective, antibacterial, antioxidant, and anti-inflammatory effectsm [28]. Artemisiae Scopariae Herba has anti-atherosclerotic and anticancer effects and increases bile secretion [29]. Schisandrae Chinensis Fructus has detoxifying, anticancer, and antioxidant effects [30]. Our pharmacological analysis showed that MAG contributes to the regulation of calcium ion transport, inflammatory factors, and antioxidant factors. Based on the anti-oxidant, anti-inflammatory, and anti-gastric secretion effects, MAG is a promising candidate for the treatment of alcohol-induced gastritis. However, these results raise other questions on the effect of MAG, including the mechanisms by which it regulates ROS-related signalling pathways.
We explored the gastro-protective effect of MAG in an alcohol-induced gastritis model. According to Kovacs et al., alcohol activates inflammatory cytokines [31]. The regulation of antioxidant proteins, such as SOD-1 and HO-1, contributes to diverse diseases, such as vascular disease, metabolic disease, gastritis, and gastric ulcer [32,33]. In particular, RNS and ROS induce deoxyribonucleic acid damage and exacerbate gastritis. Our results indicated that MAG significantly inhibits gastro-mucosal damage by upregulating SOD-1 and HO-1 in an alcohol-induced rat model. Gastritis can also be caused by infections with gram-negative bacteria, including H.Pylori, consequently increasing RNS and ROS levels via TRL2 receptor [34,35]. LPS is a major component of the outer wall of gram-negative bacteria and is a potent activator of the immune system. Furthermore, LPS induces the expression of inflammatory factors, such as iNOS, COX-2, NF-κB, and interleukins, as well as ROS generation [36]. Our results indicated that MAG significantly reduces the levels of inflammatory factors, such as iNOS, COX-2 and NF-κB. Therefore, we suggest that MAG has a gastroprotective effect against alcohol-induced gastritis via the synergistic anti-inflammatory and antioxidant effects of MAG components.
Athletes may complain of chronic gastritis through high-intensity training. According to previous studies, it has been reported that the cause of gastritis recurrence and high-intensity physical activity have a high positive correlation [37]. According to this study, as a result of surveying 63 subjects with a history of gastritis on the incidence of gastritis recurrence, most respondents (59.3%) in the category of extreme physical activity reported that they experienced intermittent recurrence. Therefore, it is suggested that more attention should be paid to the level of daily physical activity in patients to minimize the recurrence of gastritis.
It is well known that regular exercise helps by strengthening the body’s antioxidant defences. However unfamiliar, or high-intensity training can produce excess reactive oxygen species, which can lead to oxidative stress-related tissue damage and muscle contractility [38]. Interestingly, since gastritis is also known to cause reactive oxygen species, studies of natural products that can lower ROS and avoid doping problems in athletes or people with high levels of physical activity are needed. Taken together, our findings suggest that MAG is an effective candidate natural product for treating gastritis. In future studies, it is thought that additional studies are needed on the level of inflammation during high-intensity exercise after gastritis and whether long-term MAG intake can effectively suppress it.
We used a clinically relevant animal model of alcohol-induced gastritis to evaluate the effects of MAG. The four natural complexes in MAG were screened by a systematic analysis of compounds with anti-inflammatory and antioxidant effects and ion channel regulatory activity, demonstrating their ability to treat gastritis in vitro and in vivo. We demonstrated that MAG decreases the expression levels of inflammatory factors, such as COX-2, iNOS, NF-κB, and modulates the levels of antioxidant enzymes, such as SOD-1 and HO-1, in vitro and in vivo. MAG prevented alcohol-induced gastric mucosal injury. Overall, our results suggested that the components of MAG have synergistic therapeutic effects on gastritis. However, further research is needed on the decrease in gastric function through MAG and exercise, especially gastritis.
Notes
ABBREVIATIONS
ROS: Reactive oxygen species; RNS: reactive nitrate species; iNOS: inducible nitric oxide synthase; COX-2: cyclooxygenase-2; NO: nitric oxide; SOD-1: superoxide dismutase-1; HO-1: heme oxygenase-1; TCMSP: Traditional Chinese Medicine Systems Pharmacology database and analysis platform; MAG: ma-al-gan; LPS: lipopolysaccharide; OB: oral availability; DL: drug-like activity; GO: Gene Ontology; DMEM: Dulbecco’s modified Eagle’s medium; FBS: fetal bovine serum; PS: penicillin-streptomycin; ERBB2: Erb-B2 Receptor Tyrosine Kinase 2; HIF1A: Hypoxia Inducible Factor 1 Subunit Alpha; NFE2L2: Nuclear Factor and Erythroid 2 Like 2
Acknowledgements
This paper was supported by the Semyung University Research Grant of 2022. This paper was supported by the KU Research Professor Program of Konkuk University.
