Relationship between physiological tremor and cognitive function in physically active older women
Article information
Abstract
[Purpose]
This study aimed to compare the physiological tremor, grip strength, and cognitive function of sedentary and physically active older adults.
[Methods]
Twenty-four older adults aged ≥65 years participated in this study and were divided into the sedentary (76.5±4.4 years, n=12) and physically active (73.5±3.3 years, n=12) groups. Each group completed the Mini-Mental State Examination (MMSE) for cognitive function assessment. Physiological tremor was measured using an accelerometer for both hands at rest and the left/right hand with a 1,000 g dumbbell on the palm in neutral positions and the elbow flexed at 90°. Physical fitness was measured by grip strength and completion of the Short Physical Performance Battery (SPPB) and the 6-min walk test.
[Results]
The physically active group showed a significantly lower level of physiological tremor in both hands at rest and the left/right hand with a 1,000 g dumbbell on the palm (P<0.05) than that in the sedentary group. For cognitive function, the physically active group showed significantly higher scores than those in the sedentary group (P<0.001). No significant correlation was found between cognitive function and left/right grip strength (left: r = 0.117, P = 0.585; right: r = 0.230, P = 0.279), physiological tremor in both hands at rest (left: r = -0.524, P < 0.001; right: r = -0.508, P < 0.05), and the left/right hand with a 1,000 g dumbbell on the palm (left: r = -0.505, P < 0.05; right: r = -0.458, P < 0.05).
[Conclusion]
Physiological tremor of the hands has the potential to be a useful predictor of cognitive function in older adults.
INTRODUCTION
According to recent studies, adults aged ≥65 years account for 16.5% of the total population in South Korea. This percentage is predicted to reach 20.3% by 2025 and will transform South Korea into a superaged society [1]. In 2018, the life expectancy of those aged 65 years was 21.3 years (19.1 years for males; 23.4 years for females), highlighting a potential increase in health care expenditure burden and sociocultural problems [2]. Studies regarding the process of aging in relation to healthy lifestyles in older adults have been conducted in various fields [3]. It is noteworthy that some of these studies emphasized the importance of physical activity or exercise as a means of delaying to delay the aging process [4]. Manual activities in daily life, in particular, are used in a multitude of functions, whereas grip strength has been reported to predict functional limitations and disabilities in older adults that can affect their quality of life [5]. According to a previous study, aging is associated with hand tremors [6], and such tremors could lead to hand dysfunction in older adult patients with Parkinson’s disease (PD) or essential tremors [7].
Tremors are a common form of involuntary dyskinesia that arise with advancing age [8]. Tremors may reduce quality of life by obstructing activities from reading and writing to having meals. Action tremors consist of two types: kinetic and postural. Kinetic tremors occur when one gently holds a limb against gravity, while postural tremors refer to diverse tremors in daily life, including involuntary movement of muscles. The normal aging process is strongly correlated with neuromuscular system dysfunction [9]. Motor symptoms related to such dysfunction are due to an increase in the amplitude of physiological tremors in the upper limbs [10]. This may cause serious reductions in the ability to perform sophisticated motor skills during operations that require grip precision. Despite the general assumption that tremors increase as aging progresses, only a few studies have reported an increase in tremor amplitude in older adults [11,12]. The causes of tremors vary substantially. Notably, in PD patients, tremors may develop in the early stages of the disease. Over 75% of patients with PD experience tremors at rest; as the disease progresses, the symptoms are increased [13].
Cognitive dysfunction is observed in older adults as a common consequence of the aging process [14], while it is generally detected in far later stages in functionally or clinically important cases of dementia or Alzheimer’s disease [15]. The decline in cognitive ability in older adults is associated with various factors, such as hypertension, stroke, diabetes, and chronic health conditions [16]. In particular, changes in muscular strength may cause age-related changes in biological vigor. The measurement of muscular strength in relation to physical function is likely to be a useful dimension of the clinical assessment of older adults in the near future for the identification of individuals with the highest risk of persistent decreases in functional, psychological, and social health [17]. Grip strength is a marker of muscular strength, mobility, and biological capacity. In addition, the stronger the grip strength, the higher the likelihood of survival in diseases, such as cancer. Grip strength, as a simple measurement, is a relevant tool for measuring functional capacity in older adults, but it is limited to predicting neuromuscular system dysfunction with advancing age. Thus, physiological tremors at the wrist can forecast neuromuscular and cognitive functions using an accelerometer connected to a smartphone. To date, no study has investigated the association between physiological tremors and cognitive function in older adults. This study aimed to compare physiological tremors, grip strength, and cognitive function between sedentary older adults and physically active older adults.
METHODS
Participants
The participants in this study were 24 women aged ≥65 years residing in S-city, Gyeonggi-do, South Korea. Initially, 87 individuals were recruited using flyers and posted ads. Among them, 65 individuals were selected, excluding 22 who did not satisfy the inclusion criteria. A further 41 patients were excluded based on the exclusion criteria. Finally, 24 individuals were selected and placed into either the sedentary (n=12, control) or physically active (n=12, experimental) group, based on whether they habitually exercised. Those allocated to the physically active group had performed at least six months of continuous physical activity (two hours or more of aerobic exercise per week). The exclusion criteria were as follows: 1) individuals with a physical abnormality (e.g., severe arthritis or history of artificial joint replacement surgery), 2) individuals with dementia or a communicative problem, and 3) individuals with resistant hypertension, unstable angina, type I diabetes, heart failure or history of heart surgery, chronic renal failure, epilepsy, convulsion, chronic enteritis, or other diseases that may cause severe dysfunction. Prior to participation, individuals were provided with an adequate explanation of the study purpose and content, and written consent was obtained from those who voluntarily agreed to participate. This study was approved by the institutional review board (IRB: KUIRB-2019-0161-01).
Study design
Following subject selection, the selected participants were placed either into the sedentary or physically active group. After signing the voluntary agreement to participate in the consent form, the height and body composition of each participant were measured in a laboratory inside S Experience Center, located in Gyeonggi-do. The participants completed the physiological tremor test and cognitive function assessment (Mini-Mental State Examination [MMSE]), after which their physical fitness and grip strength were measured, and the Short Physical Performance Battery (SPPB) and 6-min walk test were performed.
MEASUREMENTS
Height and body composition
Height (cm) was measured using a manual extensometer (Seca; Hamburg, Germany). The participant, wearing a light garment and without shoes, was guided to stand as upright as possible with their eyes staring forward, the chin pulled slightly downward, and the eyes and ears level. Body composition was measured using a bioelectrical impedance analyzer (Inbody 270, InBody Co., Seoul, Korea).
Physiological tremor
Physiological tremor data were obtained from a sensor attached to an accelerometer that the participant wore around the wrist. To minimize unnecessary tremors and ensure a stable posture during measurement, the participants were instructed to stand vertically with their back against the wall. The wrist with the accelerometer (Accelerometers, Invernsense, Korea) was set parallel to the ground surface, based on the sensor axis. The elbow of the arm with the accelerometer was adjusted such that the accelerometer could be set to zero in the DC mode before the test in the AC mode to calibrate for body tremors. The physiological tremor was measured by an accelerometer for both hands at rest and the left/right hand with a 1,000 g dumbbell on the palm. The total testing time was 5 min and each testing session included 30 s of the recession periods. The test was repeated twice, and the average value was recorded.
Mimi-mental state examination
The MMSE was first developed by Folstein et al. (1975) to assess cognitive functions. The test consisted of 19 questions, with a total score of 30. The items and their respective scores were as follows: temporal orientation (5-point, n=5), spatial orientation (5-point, n=5), recall (6-point, n=2), attention (5-point, n=1), linguistic ability (3-point, n=2), performance (3-point, n=1), spatial arrangement (1-point, n=1), and judgement and abstract thinking (2-point, n=2). Participants with a total score of ≥24 were considered healthy; those scoring 20–33 were considered to have suspected dementia; and those scoring ≤19 were considered to have confirmed dementia.
Functional fitness
Muscular strength
Grip strength (kg) was measured as the maximum value on a hand grip dynamometer (Takei, Takei Scientific Instruments Co., Ltd, Shinagawa-Ku, Tokyo, Japan) when the participant used the left and right hands to hold the handle of the device in alignment with the second digit to pull it at maximum strength, with the arm stretched out straight at an angle of 15° to the body.
Short physical performance battery
Three items were assessed: standing balance, walking speed, and repeated chair-stand. Each of the three items was scored on a 4-point scale (from 1 to 4 points). The maximum total possible score was 12.
1. Standing balance: In the standing balance test, side-by-side, semi-tandem, and tandem stances were measured. If the side-by-side stance could be maintained for ≥10 s, a score of 1 was assigned, and the same criteria were applied to the semi-tandem stance. A score of 2 was assigned to maintain tandem stance. If the stance was maintained for <10 s or in the case of a safety risk, the test was stopped instantly, and a score of 0 was given, moving on to the test of walking speed.
2. Walking speed: In the walking speed test, the time taken to walk a 4-m distance at normal speed was measured. The same method was applied for the use of an assistive tool (such as a walking stick). A score of 1 was given for >8.7 sec, 2 for 6.21–8.7 sec, 3 for 4.82–6.20 sec, and 4 for <4.82 sec. If walking deviated from the set path or in the case of a safety risk, the test was stopped instantly, and a score of 0 was given.
3. Repeated chair stands: The test of repeated chair stands aims to measure the muscular strength of the lower limbs required for daily activities. The participant was instructed to sit on a chair and stand back-up five times with folded arms on the chest. The time was measured, and a score of 0 was given for the failure to complete the test five times within 60 sec, 1 for >16.7 sec, 2 for 13.7–16.6 sec, 3 for 11.2–13.6 sec, and 4 for <11.2 sec. If the participant touched the chair or knee with a hand or arm, or in the case of a safety risk, the test was stopped instantly, and a score of 0 was given.
Cardiopulmonary endurance test
For the 6-min walk test, a path 20 cm wide and 20 m long was prepared with the length marked by colored cones. The participant was instructed to walk back and forth along the path at the signal as fast as possible for 6 min, and the total distance walked as the displacement (m) was recorded. Participants were prohibited from running to ensure that the entire sole touched the ground while walking. At the end of 6 min, the total distance was measured in meters.
Statistical analysis
All data collected in this study were analyzed using SPSS PC+ for Windows (version 26.0; IBM, Armonk, NY, USA). The detailed data processing methods are as follows. For each measured item, descriptive statistics were estimated as means and standard deviations (SD). Normality was tested using the Kolmogorov–Smirnov test, and an independent t-test was performed to analyze between-group variation. For the correlation between grip strength and MMSE, wrist physiological tremor and MMSE, and grip strength and wrist physiological tremor, Spearman correlation analysis used as the MMSE was non-parametric. An independent t-test was used to analyze the variation in each group. The level of significance was set at ɑ=0.05.
RESULTS
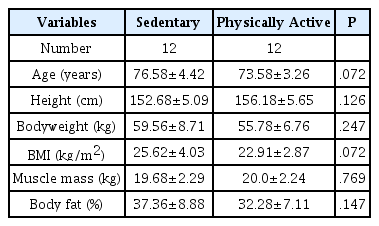
Table 1 shows the body composition of participants in each group based on their physical activity. No significant differences were found in age, height, weight, body mass index, muscle mass, or body fat ratio between the two groups.
Figure 1 shows the results of analyzing the physiological tremor on the left and right wrists at rest and with a 1,000 g dumbbell on the palm. The results indicated that, compared to the sedentary group, the physically active group had a significantly lower level of physiological tremor on the left and right wrists, both at rest and with a 1,000 g dumbbell on the palm (P<0.05).
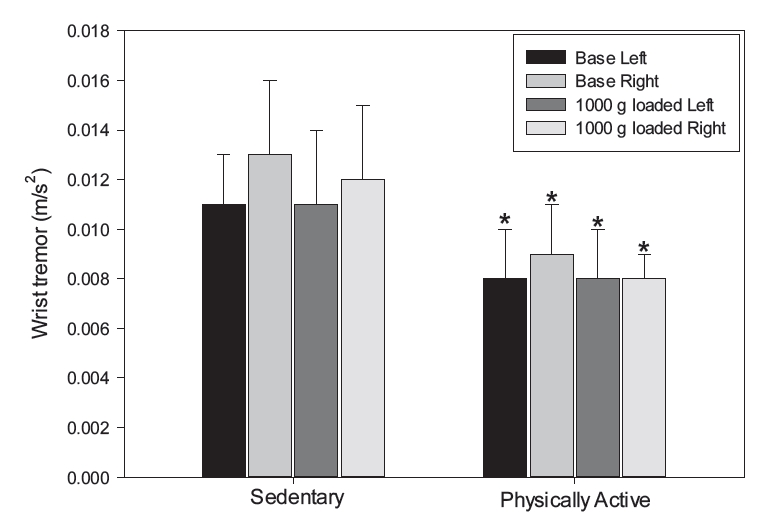
Wrist physiological tremor at the wrist between the sedentary and physically active groups. Data are presented as means±SD. Note. SD = standard deviation, Base = neutral position and the elbow flexed at 90° with the palm at rest, *P<.001 vs. sedentary.
Figure 2 shows the results of the grip strength analysis of the left and right hands using a hand grip dynamometer. The results indicated that, compared to the sedentary group, the physically active group had a trend of higher mean grip strength for both the left and right hands, although no statistically significant difference was found.
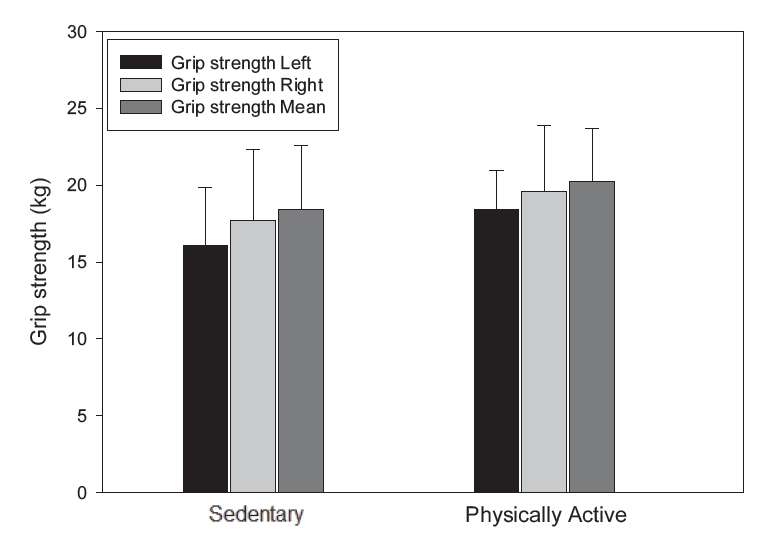
Grip strength between sedentary and physically active groups. Data are presented as means±SD. Note. SD = standard deviation.
Figure 3 shows the results of mental state analysis using the MMSE test. MMSE scores were significantly higher in the physically active group than in the sedentary group (P<0.001).
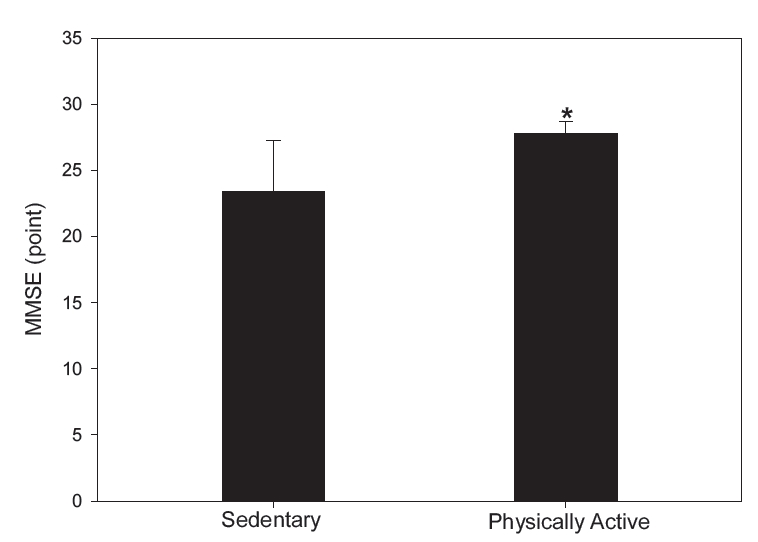
MMSE scores between the sedentary and physically active groups. Data are presented as means±SD. Note. SD = standard deviation, MMSE = Mini-Mental State Examination. *P<.001 vs. sedentary.
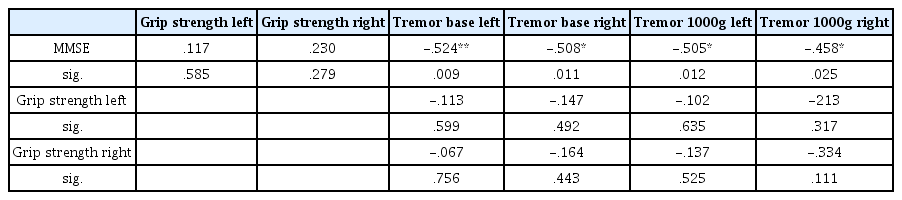
Table 2 shows the correlation analysis results for grip strength and MMSE score, wrist physiological tremor and MMSE score, and grip strength and wrist physiological tremor. No correlation was found between grip strength and MMSE scores for either left hand grip strength (r=0.117) or right hand grip strength (r=0.23) (P=0.585; P=0.279). Between wrist physiological tremor and MMSE, correlations were found for left wrist physiological tremor (r=-0.524, P<0.001) and right wrist physiological tremor (r=-0.508, P<0.05) at rest, and left wrist physiological tremor (r=-0.505, P<0.05) and right wrist physiological tremor (r=-0.458, P<0.05) with a 1,000 g dumbbell. Left hand grip strength was not correlated with left wrist physiological tremor (r=-0.113, P=0.599) and right wrist physiological tremor (r=-0.147, P=0.492) at rest, or left wrist physiological tremor (r=-0.102, P=0.635) and right wrist physiological tremor (r=-0.213, P=0.317) with a 1,000 g dumbbell. Similarly, right hand grip strength was not correlated with left wrist physiological tremor (r=-0.067, P=0.756) and right wrist physiological tremor (r=-0.164, P=0.433) at rest, or left wrist physiological tremor (r=-0.137, P=0.525) and right wrist physiological tremor (r=-0.334, P=0.111) with a 1,000 g dumbbell.
Table 3 presents the physical fitness results. Walking speed was significantly faster in the physically active group than that in the sedentary group (P<0.001). The 6 min walk test results were significantly higher in the physically active group than those in the sedentary group (P<0.001). SPPB scores were significantly higher in the physically active group than those in the sedentary group (P<0.05).
DISCUSSION
This study was conducted to investigate the relationship between physiological tremors, grip strength, and cognitive function in physically active and sedentary older adults. Twenty-four participants were divided into two groups: a sedentary group (n=12) and a physically active group (n=12). Physiological tremor, muscular strength, cognitive function, and exercise performance were analyzed. The results showed that physically active older adults had significantly higher cognitive function, physiological tremor, muscular strength, and exercise performance than sedentary older adults did. Notably, a lower level of physiological tremors indicated higher cognitive function, showing a positive correlation.
Cognitive dysfunction is commonly observed in middle-aged and older adults during the aging process [18,19]. In older adults, reduced cognitive function is closely associated with a variety of factors, including age, low level of education, low income, and chronic diseases such as hypertension, stroke, diabetes, and depression [20,21]. Among these factors, a decrease in physical fitness may lead to dysfunction of the musculoskeletal system or other systems in the body due to natural aging. Changes in muscular strength, in particular, may characterize aging with respect to biological vigor and physical function. Thus, measurement of muscular strength may be a critical clinical factor in distinguishing high-risk individuals undergoing continuous reduction in functional, psychological, and social health in the future [17].
Grip strength is not only a major indicator of muscular strength but also a marker of biological vigor; in other words, it is suitable for monitoring age-related changes in biological functions [22]. Most previous studies reported a positive correlation between cognition and grip strength. In the study by Kim et al. (2019), high levels of grip strength at rest indicated high MMSE scores as an assessment of cognitive function (β=0.14, P<0.001), in that a positive change in muscular strength could predict high MMSE scores (β=0.14, P<0.001) [19]. However, in the present study, grip strength showed no significant variation between the physically active and sedentary groups. Despite the lack of a significant difference, grip strength was relatively high in the physically active group, with significantly high MMSE scores. Little is known about the underlying mechanism of the relationship between cognitive function and physical activity. First, previous studies have reported that physical activity may have a protective effect on beta-amyloid peptide, an index of Alzheimer’s disease. Second, physical activity stimulates brain-derived neurotrophic factor (BDNF), a neurotrophin family of growth factors that are related to neural survival, activity releases myokines, such as lactate, IL-6, and irisin, in skeletal muscles. These can affect the brain function. In particular, these factors have been positively implicated in long-term memory formation [23]. As the result was obtained from a physically active group that was limited to those performing regular aerobic exercise, future studies should acquire data from a more inclusive population performing resistance exercises.
In this study, physical activity was correlated with physiological tremors and cognitive dysfunction. Many patients with essential tremor (ET) display a decline in cognitive function, from mild cognitive impairment (MCI) to dementia [24]. Moreover, cases of severe cognitive dysfunction, such as those related to concentration, memory, and frontal executive domains, are more frequently observed in patients with dementia or MCI than in patients without dementia [25]. In addition, while cognitive dysfunction is closely associated with test scores, level of education, and severity of tremors that are related to increasing age, no correlation exists with disease duration or vascular risk factors. In a study on 18 ET patients (aged 66.1±12.3 years) in the USA, no correlation was found between the severity of tremors and cognitive impairment [26]. Contrarily, a study of 16 ET patients in Turkey reported a high correlation between the severity of tremors and the Wisconsin Card Sorting Test and Hooper Visual Organization Test results, with r=0.51–0.76 [27]. However, the study also reported a lack of correlation between the severity of tremors and specific variables including concentration, memory, language, and planning. In addition, a study on 47 ET patients (aged 68.2±7.3 years) in South Korea applied neuropsychological assessments, including the Korean version of the MMSE. The participants were 17 ET patients without dementia, 21 ET patients with MCI, and 9 ET patients with dementia [28]. Contrarily, we found a significant correlation across measurements, whereby physically active and sedentary older adults were analyzed solely for physiological tremors. While the pathophysiological mechanism remains unclear, the following mechanism is predicted to underlie the correlation between tremor and cognitive function. Several recent studies have shown that the cerebellum is functionally connected to the cerebral cortex through feedforward and feedback pathways [29]. In a study of both animals and humans, the cerebellum was shown to play a critical role in tremor generation [30]. In addition, physiological tremors may also occur even in healthy individuals via abnormal proprioception feedback from the limbs (the peripheral system) to the cerebral cortex (the central nervous system) during a specific stance or performance of movements against gravity or retention of mechanical force [31]. Various studies have also reported that the fronto-subcortical circuit plays a crucial role in cognition and progression of ET in patients. Herein, we report a significant correlation between cognitive function and physiological tremor in older adults, and physiological tremor is thus anticipated to serve as a predictor of cognitive function in older adults in the future.
In this study, a significant correlation was found between cognitive function and physiological tremor in older adults; thus, physiological tremor may be suggested as a predictor of cognitive function in older adults. Furthermore, the estimation of physiological tremor of the wrist both at rest and upon isokinetic exercise with a 1,000 g dumbbell in hand is anticipated to serve as a predictor of cognitive function in older adults. Future studies should identify the causes and effects of the correlation between cognitive function and physiological tremors in accordance with physical activity.
This study had some limitations. First, as a cross-sectional study design, it cannot provide a cause-and-effect relationship. Second, owing to the relatively small number of subjects, this study may not represent the characteristics of the relationship between physiological tremors and cog nitive function in both sedentary and physically active older adults. Third, the levels of physical activity and cognitive function were measured using a self-reported questionnaire, which may be an unknown bias in the selection. Lastly, we did not fully control for variables, including education and depression, which may be critical factors for cognitive function.