Marine phytoplankton improves recovery and sustains immune function in humans and lowers proinflammatory immunoregulatory cytokines in a rat model
Article information
Abstract
[Purpose]
This study investigated the effects of marine phytoplankton supplementation (Oceanix®, Tetraselmis chuii) on 1) maximal isometric strength and immune function in healthy humans following a oneweek high-intensity resistance-training program and 2) the proinflammatory cytokine response to exercise in a rat model.
[Methods]
In the human trial, 22 healthy male and female participants were randomly divided into marine phytoplankton and placebo groups. Following baseline testing, participants underwent a 14-day supplement loading phase before completing five consecutive days of intense resistance training. In the rat model, rats were randomly divided into four groups (n=7 per condition): (i) control, (ii) exercise, (iii) exercise + marine phytoplankton (2.55 mg/kg/day), or (iv) exercise + marine phytoplankton (5.1 mg/kg/day). Rats in the exercising groups performed treadmill exercise 5 days per week for 6 weeks.
[Results]
In the human model, marine phytoplankton prevented significant declines in the isometric peak rate of force development compared to placebo. Additionally, salivary immunoglobulin A concentration was significantly lower following the resistance training protocol in the placebo group but not in the marine phytoplankton group. Marine phytoplankton in exercising rats decreased intramuscular levels and serum concentrations of tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β) and intramuscular concentrations of malondialdehyde.
[Conclusion]
Marine phytoplankton prevented decrements in indices of functional exercise recovery and immune function. Mechanistically, these outcomes could be prompted by modulating the oxidative stress and proinflammatory cytokine response to exercise.
INTRODUCTION
Muscle damage caused by intense exercise disrupts tissue homeostatic balance and causes a series of biological events, such as inflammatory responses, to initiate skeletal muscle repair [1–3]. One particular inflammatory response of interest is that of cytokines [4]. “Cytokine” is an umbrella term that refers to a group of glycoproteins produced by the immune system to facilitate communication within immune cells and between immune and non-immune cells [4]. The production of proinflammatory cytokines, such as interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) [5], is accelerated in response to inflammatory stimuli (e.g., muscle damage), and this response can be temporary or extended [4]. Cytokines are essential to the damage, repair, and remodeling processes involved in muscle tissue in response to exercise [2,4].
Additionally, intramuscular inflammation can be significantly attributed to mitochondrial uncoupling and subsequent induction of reactive oxygen species (ROS) [6], which is vastly increased during strenuous exercise [7]. Exposure to excessive free radicals can impair immune function [8] and provoke performance decrements [9,10]. Moreover, ROS can amplify the proinflammatory cytokine response to exercise [11,12], which may be deleterious and lead to maladaptation. The increase in ROS levels and subsequent elevation of proinflammatory cytokines following exercise have a temporary depressive effect on immune function, which could make individuals more susceptible to infection [13].
Salivary immunoglobulin A (sIgA) is considered the first line of defense, as sIgA binds to and opsonizes pathogenic organisms, including respiratory viruses [14]. As such, sIgA concentration has been examined as a biomarker to evaluate the immune status of athletes. Long durations of intense exercise, both acutely and chronically, decrease sIgA levels and are associated with increased respiratory infection symptoms [15]. For example, sIgA levels decrease in athletes undergoing overreaching programs and/or experiencing upper respiratory infections [16–18]. Thus, regulating pro-oxidative and proinflammatory responses could be a viable method of immunomodulation for exercise recovery.
An individual’s nutritional status has been shown to robustly influence antioxidant status, immune function, and recovery ability [19]. As a result, a fair amount of attention has been given to antioxidant supplements for exercise recovery, which is largely due to their capacity to enhance endogenous support in diminishing oxidative damage by neutralizing ROS [20,21]. The majority of studies investigating the impact of antioxidant supplementation on exercise have reported that antioxidants can reduce oxidative stress [22]; however, the physiological implications of this effect are not well known. Furthermore, strong evidence supporting antioxidant supplementation as a protectant against muscle damage is incomplete, as most investigations do not consider oxidative stress markers and proinflammatory cytokines in conjunction with functional indices of muscle damage, such as loss of muscle force and power [22,23].
Scientists have isolated a unique marine phytoplankton (MP), the microalgae Tetraselmis chuii (Oceanix®, Lonza Consumer Health Inc., Greenwood, SC, USA.). This microalgae contains highly active antioxidant enzymes, particularly superoxide dismutase (SOD), which catalyzes the conversion of superoxide into ordinary molecular oxygen, thereby protecting cells from oxidative damage [24]. Tetraselmis chuii was also found to upregulate glutathione peroxidase and catalase enzymes in human skeletal muscle myoblasts in vitro [24]. Antioxidants have been shown to aid in exercise recovery [25–28]. Mechanistic cell culture models have demonstrated a reduction in oxidative stress in response to Tetraselmis chuii exposure [24], which prompted our study design to investigate the link between oxidative stress and immune-related responses.
Thus, the current study examined the effects of microalgae Tetraselmis chuii supplementation on functional indices of muscle damage and immune function in humans following a short-term, muscle-damaging bout of anaerobic exercise. This was followed by a mechanistic rat trial to determine whether this nutritional intervention affected recovery through the production of proinflammatory immunoregulatory cytokines. We hypothesized that Tetraselmis chuii supplementation can attenuate decrements in muscle and immune function in humans and mitigate proinflammatory cytokine production and oxidative stress in exercising rats.
METHODS
Human Trial
Study Participants
Male and female participants were recruited by word of mouth, email contact, and direct website inquiries from online advertisements. Participants were excluded from the study if they had a body mass index (BMI) ≥ 30 kg/m2; had allergies to fish, shellfish, algae, or seaweed had any cardiovascular, metabolic, or endocrine disease; had undergone surgery that affects digestion and absorption; smoke or drink heavily (>7 and >14 drinks per week for women and men, respectively); were pregnant or planning to be pregnant; were on medication to regulate blood glucose, lipids, and/or blood pressure; were using anabolic-androgenic steroids; were currently using antioxidant supplements, non-steroidal anti-inflammatory drugs, or nutritional supplements known to stimulate recovery or muscle mass gains. For inclusion, participants were required to be between 18-45 years old and should have continuously exercised for the past year a minimum of 3 days per week [1], achieving 30 min of vigorous activity (≥75% age-predicted maximum heart rate) per session.
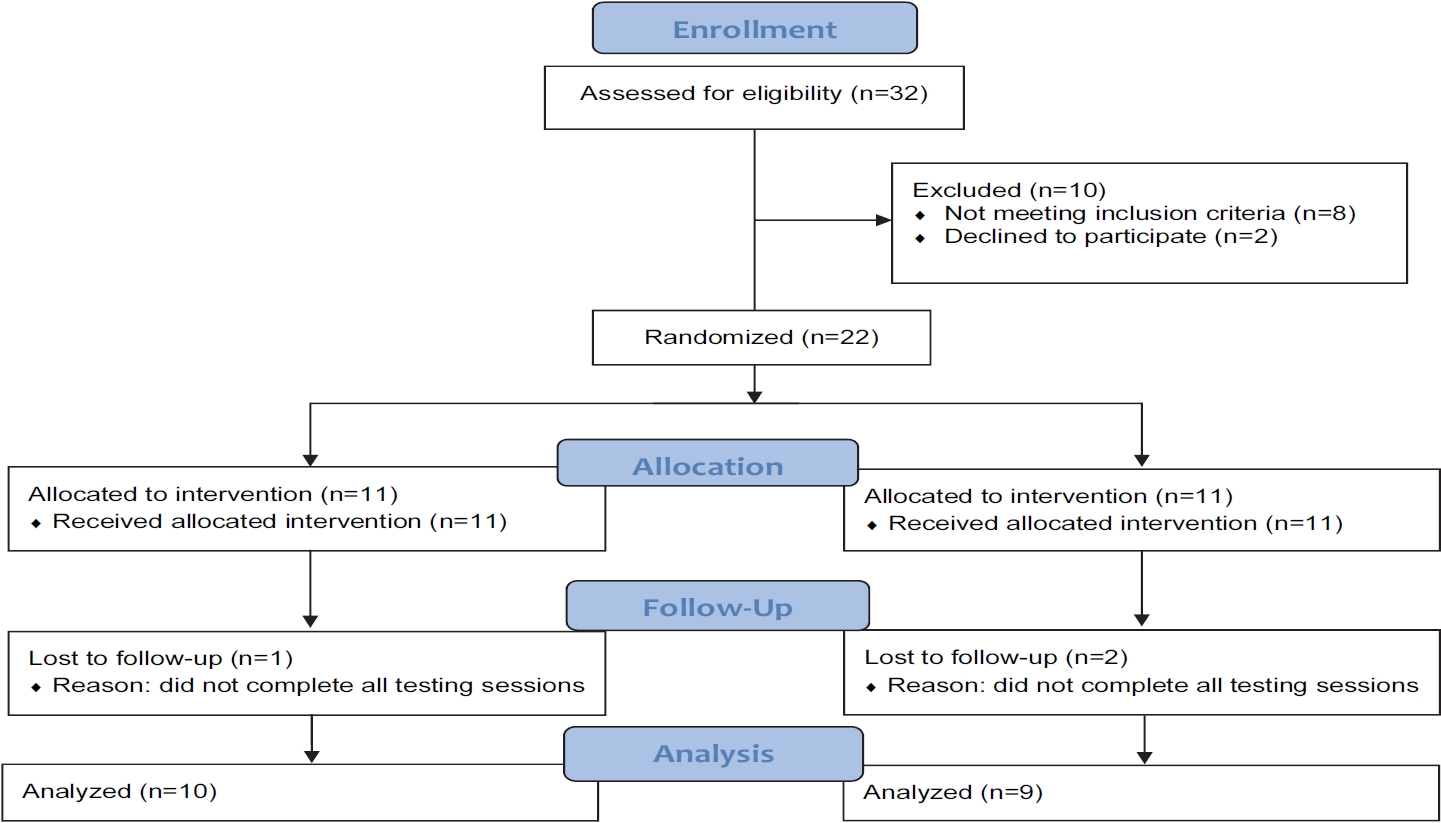
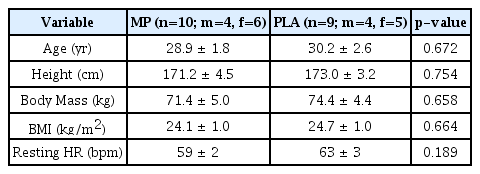
To determine the sample size for the study, an a priori power analysis (G*Power, version 3.0.10) was performed with the given α, power, and effect size values [29]. The test family was set as an F-test, and the statistical test was set as a repeated-measures analysis of variance (ANOVA) with the following parameters: α = 0.05; 1-β = 0.8; effect size = 0.5; number of groups = 2; repetitions = 4. Our previous investigation on the same MP ingredient (microalgae Tetraselmis chuii, Oceanix®) observed an effect size of 0.54 on performance metrics in the countermovement and squat jump, respectively [30]. Thus, we believe that the effect size estimation of 0.5 for the power analysis was practical. The resulting outcome parameters indicated a total sample size of 22, equating to 11 participants per group. In total, 22 participants were enrolled in the study. Nineteen of the enrolled participants completed the study, as three did not complete post-testing procedures (Figure 1). Prior to engaging in any study procedures, written informed consent for participation was obtained from all participants, which was approved by an Institutional Review Board (Protocol number: 0519; IntegReview, Austin, TX, USA), and the study was performed in agreement with the Declaration of Helsinki. This human trial has been registered at clinicaltrials.gov (NCT04315077). Descriptive statistics for baseline characteristics are provided in Table 1.
Study Protocol
This study was conducted in a randomized, double-blind, placebo-controlled, and parallel manner. Before allocation into testing groups, participants were assessed for maximal strength and explosive strength, as indicated by peak force and peak rate of force development (RFD), respectively, in a maximal effort isometric mid-thigh pull (IMTP). Participants were then stratified into quartiles based on peak force from the IMTP, and participants from each quartile were randomly divided into conditions using a random number generator (random.org). Thereafter, participants underwent baseline testing (Pre), which included the IMTP assessment and saliva sampling to assess sIgA concentration. Immediately following pre-testing, participants were administered their respective test products (Oceanix® or microcrystalline cellulose-based placebo [PLA]), and the participants underwent a 14-day supplement loading protocol. On day 15 of supplementation, the participants began a 5-day intensified resistance training protocol. The participants continued supplementation for two days after completing the supervised training protocol. Approximately 24 and 48 h after the last training session, the participants were retested in a manner identical to Pre. The study procedures are described below, and Figure 2 represents a study timeline for the human trial.
Supplement Protocol
Following random assignment, the participants were administered either MP or PLA. The treatment supplement was independently examined by Brunswick Laboratories (Southborough, MA, USA) for oxygen radical absorbance capacity (ORAC) expressed in micromole Trolox equivalent (µmol TE) per gram. The results indicated that the values were high for hydroxyl radicals at 178.71 µmole TE/gram and superoxide anions at 348.11 µmole TE/gram, moderate in peroxynitrite and peroxyl radicals at 8.65 and 29.65 µmole TE/gram, respectively, and not detectable in singlet oxygen and hypochlorite. The ORAC values for the superoxide anion corresponded with high total values (38000 IU per gram) of SOD in the raw powder. The conditions were stored in visually identical capsules and containers. Participants were required to consume one serving (25 mg) a day, either 30 min before exercise or with the first meal of the day on non-exercise days. Supplement compliance was assessed by supplement logs and collection of supplement containers, which was calculated to be 95.7 and 93.7% for MP and PLA, respectively. Participants were instructed to refrain from consuming any other nutritional supplements for the duration of the study that could confound the results.
Resistance Training Protocol
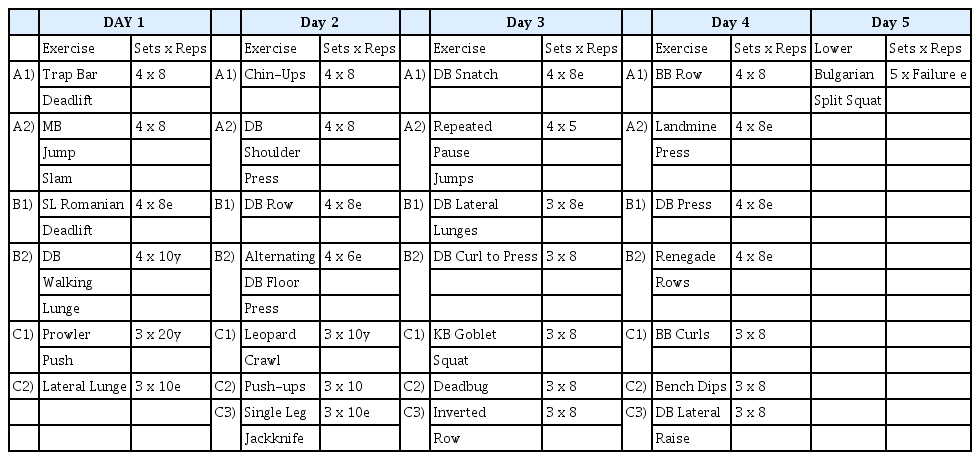
All participants completed a 5-day resistance training protocol consisting of two lower-, two upper-, and one fullbody workout. The protocol consisted of multiple supersets. Each superset consisted of 2-3 exercises (e.g., C1, C2, and C3), and each superset was performed 3-4 times before progressing to the next superset. Once Superset 1 was completed, participants moved to Superset 2 and so forth. Up to 2 min of rest was allowed between the supersets. Minimal rest was given between exercises within a superset, and participants were instructed to attempt to finish each superset as quickly as possible. All sets performed during the first four days of the protocol were performed with repetition maximum loads such that sets were performed to momentary concentric failure. Whenever participants missed the targeted repetition count by two or more repetitions, the load was decreased by 5%, and whenever participants surpassed the targeted repetition count by two or more, the load was increased by 5%. On day 5, the participants performed straight sets of Bulgarian split squats to failure for a total of 10 sets (5 sets on the right and left legs). Sets were alternated between legs and were separated by 1 min of rest. Volume was tracked by counting the total number of repetitions on the Bulgarian split squats. To set up the exercise, participants were instructed to place one foot on the ground with the other foot elevated on a pad directly behind them. The participants descended downward to a 90° angle at the knee and ascended upward to complete a repetition. The participants were instructed to only ascend or descend in response to an audible metronome beep at a cadence of 1 s downward and 1 s upward. Participants were instructed to complete as many repetitions as possible until concentric muscular failure, which was defined as failure to complete two successive repetitions at the prescribed metronome cadence. The basis of this 5-day resistance training protocol was to induce muscle fatigue to access recovery. Completing loaded sets to a repetition maximum or momentary concentric failure, as implemented in this protocol, can induce muscle fatigue [31–33]. The participants performed a dynamic warm-up before each training session. Immediately following the completion of all resistance training sessions, participants completed a cool-down consisting of static stretching of the major muscle groups targeted within the resistance training session. All stretches were repeated twice and held for 20 s each. All warm-up, resistance training, and cool-down sessions were supervised by a certified strength and conditioning specialist (NSCA-CSCS), who also monitored training loads for each exercise [34]. Whenever five or more participants were on the training floor at once, two certified strength and conditioning specialists supervised the training floor, and a maximum of 10 participants was allowed on the training floor on days 1-4. On day 5, a maximum of five participants was allowed on the floor, and two certified strength and conditioning specialists were on the training floor at all times during the protocol. All training sessions were completed with at least 24 h of recovery from the previous training session. A description of the resistance training protocol is provided in Table 2.
Maximal IMTP
Each participant was tested for maximal isometric strength and peak explosive strength in the IMTP performed in an Olympic-style half rack to allow fixation of the bar at any height. Participants were secured to the bar using lifting straps and athletic tape. Utilizing a pronated clean grip, participants were instructed to assume a body position similar to the second pull of the snatch and clean. The knee angle was confirmed between 125-135° using a hand-held goniometer, and the hip angle was set at approximately 175°. Once the body positioning was stabilized, each participant was given a countdown. Minimal pre-tension was allowed to eliminate slack prior to IMTP initiation. Each participant performed two warm repetitions, one at 50% and the other at 75% of the perceived maximum effort. Thereafter, participants completed two maximal IMTP lasting 6 seconds, separated by 2-3 minutes rest. Participants were instructed to pull fast and hard and were given strong verbal encouragement during the assessment. Strength was determined by measuring the peak force attained during the IMPT pull. Peak explosive strength was determined as the peak rate of force development (RFD) during the maximal pulling movement. Force production was recorded using a linear position transducer [35].
sIgA Levels
Salivary immunoglobulin A samples were collected using IPRO Oral Fluid Collector (OFC) kits (Soma Bioscience; Wallingford, UK). The OFC kits collect 0.5mL of oral fluid and contain a color-changing volume adequacy indicator within the swab, giving collection times typically in the range of 20-50 s [36]. All samples were collected following a 10 h overnight fast. The samples were analyzed using an IPRO POC lateral flow device (LFD; Soma Bioscience; Wallingford, UK), specific for sIgA, in an IPRO LFD Reader. Two drops of saliva/buffer mix from the OFC were added to the sample window of the LFD. The liquid runs the length of the test strip via lateral flow, creating control and test line visible in the test window. Ten minutes after the sample was added, the test line intensity was measured in an IPRO LFD Reader and converted to a quantitative value.
Rat Trial
Rat Study Protocol
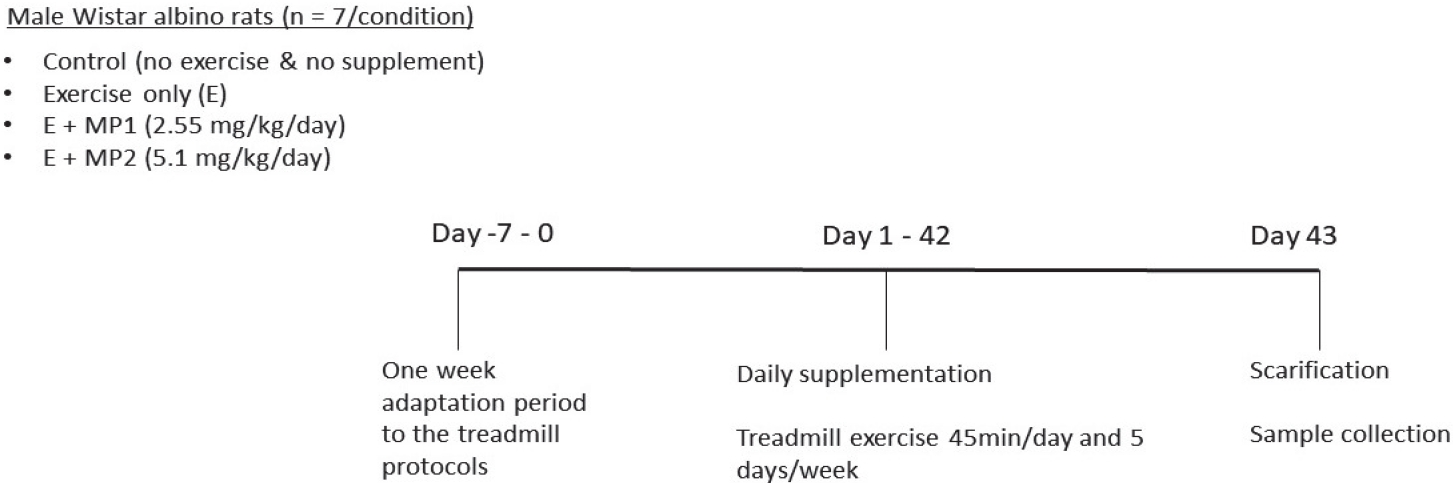
The protocol included 8-week-old male Wistar albino rats with a body mass between 200-250 g and free of any detectable disease. Rats (n= 28) were obtained from the Laboratory Animal Research Center, Firat University (Elazig, Turkey). Rats were kept in identical cages in the same room under standard conditions (22 ± 2°C temperature, 55 ± 5% humidity, 12 h light/dark cycle). Ethical permission for the experiment was obtained from the Animal Experimentation Ethics Committee of Firat University (2019/139–206) according to the relevant laws, guidelines, and restrictions.
A random number generator (random.org) was used to randomly assign rats to one of four possible conditions in a blinded fashion: (i) control (no exercise and no supplement [CON]), (ii) exercise only (E), (iii) exercise + marine phytoplankton 1 (2.55 mg/kg/day, [E + MP1]), and (iv) exercise + marine phytoplankton 2 (5.1 mg/kg/day [E + MP2]). A total of seven rats were assigned to each condition, and body mass was not significantly different between the conditions (p>0.05; mean ± SEM: Con = 207.3 ± 4.9 g, E = 217.7 ± 7.6 g, E + MP1 = 211.7 ± 9.8 g, E + MP2 = 214.6 ± 11.5 g). Marine phytoplankton and vehicle (physiological saline) were administered orally via gavage daily before exercise during the experimental period (6 weeks).
Rats in exercising conditions were subjected to treadmill exercise on a motorized rodent treadmill (Commat Limited, Ankara, Turkey). The treadmill contained a stimulus grid at the back end of the treadmill, providing an electric shock when the animal placed its paw on the grid. The apparatus had a 5-lane animal exerciser, utilizing a single belt unit divided by walls suspended over the tread surface. To eliminate diurnal variations, all exercise tests were performed during the same time of the day. A week of adaptation was provided as a pre-training practice for the animals to familiarize themselves with the treadmill equipment and handling. To do so, the rats in the exercise training groups were accustomed to treadmill exercise over a 5-day period: (i) day 1, 10 m/min, 10 min; (ii) day 2, 20 m/min, 10 min; (iii) day 3, 25 m/min, 10 min; (iv) day 4, 25 m/min, 20 min; and (v) day 5, 25 m/min, 30 min. After adaptation for a week to the treadmill system to assess the novel and stress impacts, the rats in treadmill exercise groups ran on the treadmill at 25 m/min, 45 min/day, and five days per week for 6 weeks according to the protocol described by Liu et al. [37]. Figure 3 shows the study timeline for the rat trial.
Sample Collection
At the end of the study, animals in all groups were sacrificed by cervical dislocation under xylazine (10 mg/kg, i.m.) and ketamine (50 m/*kg, i.m.) anesthesia on the same day; thereafter, blood and gastrocnemius muscle were collected. Serum samples were obtained by collecting blood samples in biochemical gel tubes after centrifugation (5000 rpm at 4°C for 10 min). Samples of the gastrocnemius muscle (taken from approximately the same location each time) were quickly removed, placed on ice, and stored at -80°C until further analysis.
Biochemical Analysis
Serum concentrations of biochemical parameters were assayed using a portable automated chemistry analyzer (Samsung LABGEO PT10, Samsung Electronics Co., Suwon, Korea). Interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNFα) kits were used to analyze the levels of these parameters (Linco Research Inc., St. Charles, MO, USA) with an ELISA instrument (Elx-800, Bio-Tek Instruments Inc., Vermont, USA).
Malondialdehyde (MDA) concentration in muscle tissue was measured using an HPLC device with a Shimadzu UV–Vis SPD-10 AVP detector, a CTO-10 AS VP column, and a mobile phase comprising 30 mM KH2PO4 and methanol (82.5: 17.5, v/v) at a flow rate of 1.2 mL/min. Tissue homogenates (10%, w/v) were prepared in 10 mM phosphate buffer and centrifuged at 13.000 × g for 10 min at 4 °C.
Western Blot Analysis
Muscle samples were pooled and homogenized in 1 mL of ice-cold hypotonic buffer A containing 10 mM HEPES (pH 7.8), 10 mM KCl, 2 mM MgCl2, 1 mM DTT, 0.1 mM EDTA, and 0.1 mM phenylmethylsulfonyl fluoride (PMSF). Eighty microliters of 10% Nonidet P-40 (NP-40) solution was added to the homogenates, and the mixture was then centrifuged for 2 h at 14,000 × g. Buffer A (500 µL) plus 40 µL of 10% NP-40 was used to wash the precipitates containing nuclei. The precipitates were then centrifuged and resuspended in 200 µL of buffer C [50 mM HEPES (pH 7.8), 50 mM KCl, 300 mM NaCl, 0.1 mM EDTA, 1 mM DTT, 0.1 mM PMSF, and 20% glycerol], and centrifuged for 30 min at 14.800 × g. The supernatant was then transferred to a new tube. Western blot analyses were performed on tissue homogenates for IL-1β and TNF-α. Protein concentration was measured using the Lowry method. A pool of tissue samples was created with the same amount of protein (50 µg) and the samples were electrophoresed on 12% SDS-PAGE gels, followed by transfer to a nitrocellulose membrane (Schleicher and Schuell Inc., Keene, NH, USA). The primary antibody for β-actin was delivered (Abcam Inc., UK) and diluted (1:1000) in the same buffer containing 0.05% Tween-20. The nitrocellulose membranes were incubated overnight at 4°C. After washing, the blots were incubated with goat anti-mouse IgG (horseradish peroxidase-conjugated secondary antibody) at a dilution of 1:5000 (Abcam, Cambridge, UK). Protein bands were quantified by scanning densitometry using an image analysis system (ImageJ; National Institutes of Health, Bethesda, USA). The protein bands were normalized to the corresponding β-actin band values and compared with those of the control group.
Statistical Analysis
Before performing statistical analysis, data were tested for normality and variance using the Shapiro-Wilk test and Levene’s test, respectively. A mixed model analysis of variance (ANOVA) was performed assuming group (MP and PLA) as the between-participant factor, time (Pre, 24 h-Post, and 48 h-Post) as the within-participant factor, and participants as a random factor. No gender effects were observed on any reported dependent variable; therefore, the data were collapsed. In the rat trial, one-way ANOVA was performed. When a significant F value was obtained, post-hoc testing with Tukey’s adjustment was performed for multiple comparisons. The absolute mean difference (Time2 – Time1) was analyzed to compare groups with unpaired, two-tailed t-test for select variables (peak RFD). Additionally, we reported the mean difference and upper and lower limit values of 95% confidence intervals (95% CI) of the mean difference for within-group comparisons, as this approach allows variable change due to supplementation to be investigated, rather than only the level of statistical significance. In this regard, the confidence interval includes the value range in which the true population mean of the difference is likely to be contained. Confidence intervals that did not contain zero were considered significant at p<0.05 [38]. Lastly, within-group effect sizes (ES) were calculated as [(Mean2 – Mean1)/Pooled SD). For all analyses, the significance level was set at p≤0.05. Results are expressed as the mean and standard error of the mean (SEM) unless otherwise noted. Data were analyzed using SPSS software (IBM SPSS, Version 26.0; SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 8 software (GraphPad Software, Inc., La Jolla, CA, USA).
RESULTS
Human Trial
IMTP
No significant differences existed between groups at Pre for IMTP peak force (p=0.832) and peak rate of force development (RFD, p=0.519). No significant main effects or interactions were detected for peak force (p>0.05). A significant main effect of time was indicated for peak RFD (p=0.007). However, confidence interval analysis suggested that only the PLA group experienced significantly decreased peak RFD at 48 h-Post compared to 24 h-Post (PLA: meandiff= -1908, 95% CI= -3768 to -48 N * s-1, ES = 0.46, p=0.043; MP: meandiff= -949, 95% CI= -2713 to 688 N * s-1, ES=0.04, p=0.306) and Pre (PLA: meandiff= -2202, 95% CI= -4062 to -342 N * s-1, ES = 0.51, p=0.017; MP: meandiff= -949, 95% CI= -2713 to 688 N * s-1, ES=0.31, p=0.396; Figure 4A). Additionally, the absolute mean difference from Pre to 48 h-Post in peak RFD was significantly different between groups (PLA: -2202 ± 981 vs. MP: -949 ± 716 N * s-1, p≤0.05; Figure 4B).
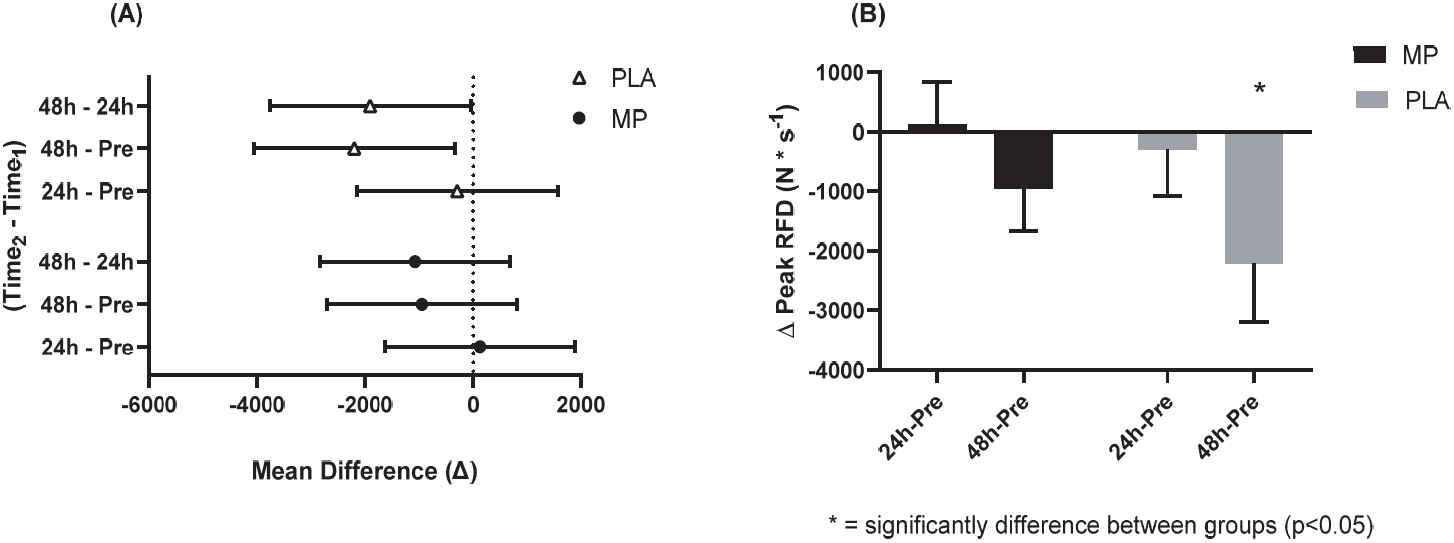
IMTP Peak Rate of Force Development at 95% CI Difference (A) and Delta Change (B).
The 95% CI of the mean difference of Time2 – Time1 (A) and the delta change from Pre values (B) for IMTP peak RFD. Figure 4A shows the mean with the upper and lower limits of the 95% CI. Figure 4B shows a bar chart for the mean values and error bars for the standard error of the mean. * = significantly different between conditions (two-tailed, unpaired t-test; p<0.05). The conditions were marine phytoplankton (MP) and placebo (PLA). Abbreviations: IMPT, isometric mid-thigh pull; RFD, rate of force development; CI: confidence interval.
sIgA Levels
No significant differences were observed between the groups at Pre for sIgA (p=0.863). A significant main effect of time was detected for sIgA (p=0.004). Confidence interval analysis suggest that only the PLA group demonstrated significantly lower sIgA from Pre to 48 h-Post (PLA: meandiff= 202.8, 95% CI= 26.7 to 379.0 µg/mL, ES: 1.12, p=0.021; MP: meandiff= 148.8, 95% CI= -22.9 to 316.0 µg/mL, ES: 0.66, p=0.108; Figure 5).

Salivary IgA Concentration at 95% CI Difference (A).
The 95% CI of the mean difference of Time2 – Time1 (A) and concentration values (B) salivary immunoglobulin A. Figure 5A shows the mean with the upper and lower limits of the 95% CI. Figure 5B shows a bar chart for the mean values and error bars for the standard error of the mean. † = significantly different from Pre per confidence interval interpretation (p<0.05). The conditions were marine phytoplankton (MP) and placebo (PLA). Abbreviations: sIgA, salivary immunoglobulin A; CI: confidence interval.
Exercise Protocol Training Volume on Day 5
A significant main effect of set was detected (p<0.001; Figure 6), whereby both groups completed significantly lower repetitions on sets 2-5 compared to set 1 (meandiff= -46 to -40 repetitions, p<0.001). The unpaired t-test indicated no significant differences (p=0.918) between groups for total training volume during the (muscle failure) training session on day 5 (mean total repetitions per participant: MP = 250 ± 112, PLA = 243 ± 149; group total repetition sum: MP = 2495, PLA = 2189).
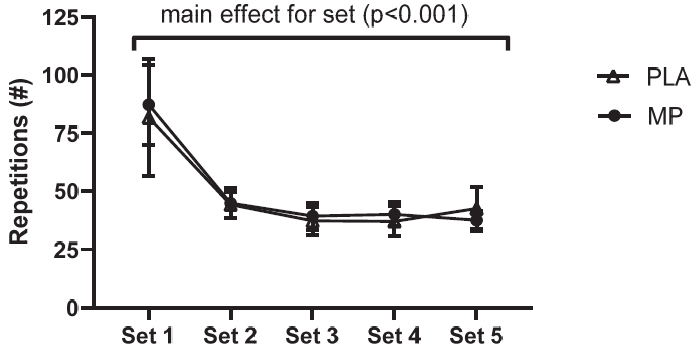
Repetition Count per Set.
The repetition count for each set of the day 5 exercise protocol. The counts are the sum of repetitions completed on the left and right legs. The Figure 6 shows a line graph with the mean values and error bars for the standard error of the mean. The conditions were marine phytoplankton (MP) and placebo (PLA).
Rat Trial
Levels of IL-1β and TNF-α
No significant differences were noted between the CON and E arms for IL-1β levels (p>0.05), whereas both E+MP1 (meandiff= -0.23 au, ES: 2.19, p<0.01) and E+MP2 (meandiff= -0.45 au, ES: 7.36, p<0.001) demonstrated significantly lower IL-1β levels compared to CON. Additionally, E+MP2 revealed significantly lower IL-1β levels compared to the exercise and E+MP1 arms (meandiff= -0.22 au, ES= 2.34, p<0.01; Figure 7A). TNF-α levels were significantly lower than CON in E (meandiff= -0.19 au, ES= 2.12, p<0.01), E+MP1 (meandiff= -0.35 au, ES= 4.58, p<0.001), and E+MP2 (meandiff= -0.47 au, ES= 6.21, p<0.001). Furthermore, TNF-α levels in both supplement arms were lower than those in the exercise arm (E+MP1: meandiff: -0.16 au, ES: 2.02, p<0.01; E+MP2: meandiff: -0.28 au, ES: 3.57, p<0.001), and E+MP2 was lower than E+MP1 (meandiff: -0.12 au, ES: 1.86, p<0.05; Figure 7B). Figure 7 displays the mean and SEM of these results.
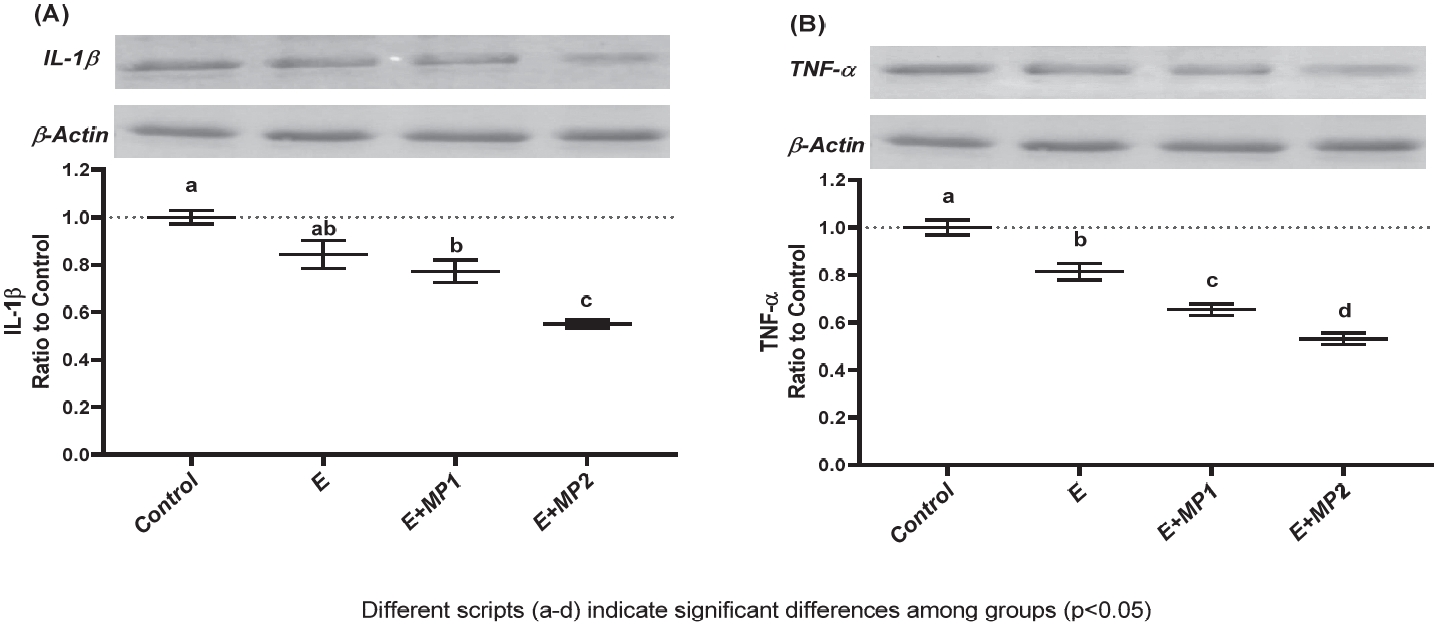
Levels of Muscle IL-1β (A) and TNF-α (B).
Mean and standard error for intramuscular IL-1β (A) and TNF-α (B) levels in rats following 6 weeks of motorized treadmill exercise. The densitometric analysis of the relative intensity according to the control group of the western blot bands was performed with β-actin normalization to ensure equal protein loading. Different symbols (a-d) indicate statistical differences among the groups (ANOVA and Tukey's post-hoc test; p<0.05). The conditions were as follows: control (CON), exercise (E), exercise + marine phytoplankton 1 (2.55 mg/kg/day, [E + MP1]), and exercise + marine phytoplankton 2 (5.1 mg/kg/day [E + MP2]). Abbreviations: IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-alpha.
Regarding IL-1β serum concentration, no significant differences were noted between the CON, E, and E+MP1 groups (p>0.05). However, IL-1β concentration was lower in E+MP2 compared to that in CON (meandiff: -3.14 pg/mL, ES: 2.47, p<0.01) and E (meandiff= -3.40 pg/mL, ES: 2.32, p<0.01) but not significantly different than that in E+MP1 (p>0.05). For TNF-α concentration, levels were significantly lower in E+MP1 and E+MP2 compared to those in CON (E+MP1: meandiff= -3.13 pg/mL, ES: 3.55, p<0.01; E+MP2: meandiff= -4.64 pg/mL, ES: 4.43, p<0.001) and E (E+MP1: meandiff= -1.83 pg/mL, ES: 1.32, p<0.05; E+MP2: meandiff= -3.34 pg/mL, ES: 2.23, p<0.01). Figure 8 displays the mean and SEM of these results.

Serum Concentrations of IL-1β (A) and TNF-α (B).
Mean and standard error for serum concentrations of IL-1β (A) and TNF-α (B) in rats following 6 weeks of motorized treadmill exercise. Different symbols (a-d) indicate statistical differences among the groups (ANOVA and Tukey's post-hoc test; p<0.05). The conditions were as follows: control (CON), exercise (E), exercise + marine phytoplankton 1 (2.55 mg/kg/day, [E + MP1]), and Exercise + marine phytoplankton 2 (5.1 mg/ kg/day [E + MP2]). Abbreviations: IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-alpha.
Muscle MDA
Muscle MDA concentration was lowered by exercise and exercise plus supplementation compared to CON (E: meandiff= -0.24 nmol/mg, ES= 2.00, p<0.05; E+MP1: meandiff= -0.46 nmol/mg, ES: 3.84, p<0.01; E+MP2: meandiff= -0.69 nmol/mg, ES: 5.23, p<0.001). Exercise plus supplementation lowered MDA concentration more than E alone (E+MP1: meandiff= -0.22 nmol/mg, ES: 2.02, p<0.05; E+MP2: meandiff= -0.45 nmol/mg, ES: 3.76, p<0.01), and levels in E + MP2 were lower than E + MP1 (meandiff= -0.23 nmol/mg, ES= 1.92, p<0.05; Figure 9).

Muscle MDA Concentration.
Mean and standard error for muscle MDA concentration in rats following 6 weeks of motorized treadmill exercise. Different symbols (a-d) indicate statistical differences among the groups (ANOVA and Tukey's post-hoc test; p<0.05). The conditions were as follows: control (CON), exercise (E), exercise + marine phytoplankton 1 (2.55 mg/kg/day, [E + MP1]), and exercise + marine phytoplankton 2 (5.1 mg/kg/day [E + MP2]). Abbreviations: MDA, malondialdehyde.
DISCUSSION
The primary purpose of this study was to examine the effects of MP supplementation on functional indices of muscle damage and, secondarily, its underlying impact on immune function in a human model following a short-term, muscle-damaging bout of anaerobic exercise. The primary purpose of the rat model was to mechanistically investigate if MP could lower the proinflammatory immunoregulatory cytokine response. Secondarily, we sought to determine if the proinflammatory cytokine response is mediated by reduced oxidative stress. We hypothesized that the ingredient would mitigate decrements in functional indices of muscle damage and preserve immune function following a challenging short-term intensified exercise protocol. Mechanistically, we hypothesized that MP would decrease proinflammatory cytokines and oxidative stress relative to the control. The primary findings of this study support our hypotheses. Specifically, MP supplementation blunted increases in functional indices of muscle damage and prevent the decline in immune function, as indicated by sIgA levels. Mechanistically, we found that the administration of MP lowered intramuscular oxidative stress with a concomitant decline in proinflammatory cytokines compared to the control.
The phenomenon of contraction-induced skeletal muscle damage, also referred to as minor contractile-induced injury, has received considerable interest in the medical literature. Physical activity inducing mechanical overload, muscle stretching, or both have been causative factors of muscle damage [39–42], which has been exhibited by structural disruption of myofilaments [43], sarcolemma damage [44], loss of muscle fiber integrity [44], leakage of muscle proteins into the blood [44,45], and acute inflammatory responses [3,46]. However, far less is known regarding the implications of these physiological associations of muscle damage on functional indices (i.e., muscle force parameters). From both practical and clinical science perspectives, the damage criteria should be related to muscle function. Rate of force development, also referred to as explosive strength, has gained strong support as a functional indicator of muscle damage [47]. Explosive strength is the speed or rate at which an individual can develop force [48]. This motor ability predicts performances of daily functional tasks to a greater degree than maximal voluntary contraction, which is seldom expressed in the time constraints of daily activity [48]. Moreover, this measure has been determined to be more sensitive to detect acute (24-72 h) [47] changes in neuromuscular function as compared to nontime-constrained maximal contraction measures. While this study consisted of intense and high-volume exercise, our results indicated that maximal strength was sustained. However, explosive strength in the PLA group was significantly lower at 24 (-27%) and 48 (-30%) h following the muscle-damaging event compared to Pre levels. Additionally, the absolute mean difference in explosive strength from Pre to 48 h-Post training was lower in the PLA group than in the MP group.
Exercise-induced fatigue can occur both peripherally and centrally. Peripheral fatigue occurs primarily in skeletal muscle and is exacerbated by the depletion of energy stores, accumulation of metabolic by-products, and muscle damage from mechanical and chemical disturbances [49]. In contrast, central fatigue is simply considered a reduction in the ability to maximally activate a given muscle [50]. This type of fatigue can be mediated by group III and IV afferent feedback loops [51], increased BCAA metabolism [52], and increased motor cortex excitability [50].
The impairment in explosive strength performance combined with the reduction in time to reach maximal force allowed us to theorize that A) the exercise stimulus was sufficient to induce neuromuscular (central) and peripheral fatigue (damage), and B) the participants completed the post-testing protocol in a state of neuromuscular and/or peripheral fatigue. This hypothesis follows the findings that explosive strength can better detect acute (24-72 h) [47] changes in neuromuscular function as compared to non-time-constrained maximal strength measures. These findings also indicate that MP can sustain anaerobic performance and improve recovery following short-term, high-intensity training.
A new paradigm in exercise physiology posits close communication between immune responses and recovery kinetics [53]. Fragala et al. [53] suggested that physical exercise elicits a system-wide immune response that is directed at restoring homeostasis. When this response is interrupted, recovery is hindered [26,54]. Studies have reported that immune function can be diminished following acute bouts of heavy repeated exercise [13,55,56], and individuals engaged in intensive periods of high-intensity training are more susceptible to minor infections [54,56]. For example, according to various surveys, impaired immune function is more common in athletes than in the general population, and infection may last longer in athletes [8,55,57]. This is a concern for trainees, as it is generally recognized that weakened immune function can result in a decrease in exercise performance and the ability to sustain heavy training [54]. Additionally, infections can be associated with the development of chronic fatigue [58], and training during periods of severe infection can increase the risk of overtraining [59]. Previously, in American football players, the incidence of upper respiratory tract infection increased during periods of intense exercise, while sIgA concentration simultaneously decreased [18]. Likewise, in a study on elite swimmers, sIgA was significantly depressed over a seven-month training period [60]. Both the aforementioned studies demonstrated significant inverse correlations between sIgA concentrations and infection rates. The present study found that sIgA was significantly reduced in PLA but was maintained in MP following a one-week resistance training protocol. Although speculative, these findings indicate that MP supplementation may improve recovery through an antioxidant immunomodulatory mechanism, which we elaborate on through our mechanistic rat model.
To achieve cellular homeostasis, a balance between ROS production and antioxidant capacity is required. It has been proposed that cellular redox homeostasis is governed by redox-sensitive signaling mechanisms, which react to the amplified formation of ROS by stimulating oxidant scavenger systems [61]. While the immune system coordinates such mechanisms to defend against deleterious pro-oxidant actions in basal conditions, the system may not react sufficiently in moments of rapid and exaggerated ROS formation. For instance, skeletal muscle generates ROS at an accelerated rate during contraction [62]. During bouts of muscle failure, an influx of calcium ions in the cytoplasm due to the overloaded mitochondria provoke greater concentrations of ROS in the cytoplasm [63]. In turn, this stimulates the release of proteases, such as calpains, that degrade muscle proteins, which subsequently increases the permeability of the muscle cell membrane [63]. Additionally, ROS have been shown to directly contribute to muscle damage [64], which potentially occurs through the peroxidation of phospholipids in the muscle cell membrane [65]. Previous research has confirmed that increased ROS production in response to exercise can cause oxidative modifications of nucleic acids, lipids, proteins, and multiple cellular compounds [66–68]. A significant portion of exercise-induced inflammation within the muscle can be traced to mitochondrial uncoupling and subsequent induction of ROS [6]. Thus, mechanistically, the contractile-induced inflammatory processes may underlie impaired immunity, at least acutely, as amplified ROS production could cause oxidative and muscle damage, prompt immune cell death, and increase susceptibility to infection [6,13].
Formation of ROS has been shown to increase proinflammatory cytokine expression [12] by activating nuclear factor-kappa β (NF-kβ) [69–72]. Activated NF-κB moves from the cytosol and relocates into the nucleus of cytokine-producing cells, increasing the transcription of cytokine mRNA [73]. Proinflammatory cytokines, such as IL-1β and TNF-α, orchestrate widespread metabolic changes and modulate immune system activity, enhancing their production [73]. Proinflammatory cytokines induce and mediate catabolic activities [74], some of which are regulators of the ubiquitin-proteasome pathway targeting protein degradation [75]. Additionally, proinflammatory cytokines impede the function and expression of IGF-1 [76], a local anabolic growth factor involved in protein anabolism. Collectively, these results suggest that proinflammatory cytokines can interfere with skeletal muscle recovery following exercise.
Nutrients can profoundly affect cytokine biology [73,77]. Previous research has shown that antioxidant supplementation can impact immune function in response to exercise by neutralizing ROS [78–80]. Other studies have supported the impact of antioxidant supplementation on immune parameters [81,82]. Further research indicates that supplementation with a combination of antioxidants attenuates exercise-induced inflammatory responses [83,84]. Similarly, supplementation of some ergogenic aids, such as creatine monohydrate and β-hydroxy-β-methylbutyrate, has demonstrated a reduction in proinflammatory cytokines post-exercise [85–87].
The ingredient administered in this study was freezedried Se, Tetraselmis chuii. This microalgae has highly active antioxidant enzymes, namely superoxide dismutase (SOD), which catalyzes the conversion of superoxide into molecular oxygen, thereby reducing the risk of oxidative cell damage [24]. The ingredient was also found to upregulate glutathione peroxidase and catalase enzymes in human skeletal muscle myoblasts in vitro [24]. Studies have shown that exercise-induced muscle damage can be reduced with acute [88,89] and chronic [90–92] antioxidant supplementation, and consequently, attenuate the inflammatory cytokine response [83]. An independent analysis of the present ingredient demonstrated high antioxidant capacity via high concentrations of SOD (38,000 IU per gram) with robust corresponding ORAC values for the corresponding anion of the enzymes. These properties led us to explore the impact of the ingredient on muscle oxidative stress and the ensuing proinflammatory cytokine response. We observed a reduction in muscle MDA, an indicator of oxidative stress, and a reduction in the proinflammatory cytokine response of IL1β and TNF-α. These proinflammatory markers contribute to muscle catabolism and may affect contractile properties and diminish the function of the neuromuscular junction [93,94]. Consequently, it is plausible that mitigating proinflammatory cytokines could concomitantly improve the function of the neuromuscular joint and subsequently improve the functional capacity of the musculature. This cascade of events has been previously proposed [95].
There are some noteworthy limitations to this study. Participants were instructed to maintain their typical eating habits (e.g., eat the same number of meals with the same meal schedule) and avoid drastic changes in diet types. However, dietary intake was not tracked; therefore, nutrition data could not be provided. Additionally, other habits potentially affecting exercise recovery, such as sleep and non-exercise-related activity outside of the laboratory, were not monitored. Next, in the human model, we did not measure common hormonal or oxidative stress biomarkers used in previous research to evaluate exercise-related stress or recovery [9,96–98]. Lastly, the training volume was tracked only on the final training session (day 5) by counting completed repetitions in a lower-body muscle failure protocol. However, considering that both groups had similar force generation capacity at Pre and accumulated similar volume on day 5, it is conceivable that a similar training status existed between groups that could prompt parallel volume loads for the entire protocol.
In the human trial, we can conclude that the exercise stimulus was effective at inducing high levels of muscle damage, as indicated by reductions in muscle force indices and induced immunosuppression. Supplementation with the microalgae Tetraselmis chuii improved short-term recovery, reduced functional muscle damage, and better preserved immune function during the fatiguing period. We have shown that microalgae supplementation can preserve immune function following intensive training, thus initiating a more favorable environment for recovery and adaptation in humans. Second, we present mechanistic data in a rat model supporting the role of microalgae in modulating the proinflammatory cytokine response and reducing oxidative stress during exercise training. These findings shed light on a plausible nutritional intervention provoking immunomodulatory effects that may be involved in enhancing exercise recovery following intense, fatiguing protocols.
Acknowledgements
This study was financially supported by Lonza Consumer Health Inc. S.D. and Z.S. were employed by Lonza Consumer Health Inc. Neither of these authors were involved in data collection, data curation, or formal analysis. All other authors have no competing interests to declare.