|
 |
- Search
| Phys Act Nutr > Volume 24(3); 2020 > Article |
|
Abstract
[Purpose]
Although physical activity is required to prevent or ameliorate osteoporosis, medicine prescription should precede it, since it may be limited in severe osteoporosis patients. Furthermore, osteoporosis has a great effect on physical activity disorders that accompany fractures and pain, and therefore, research on treatment or prevention to decrease the number of patients is required. The purpose of this study was to discover candidate substances from natural products with an effective pharmacological action and to prepare basic data to help patients.
[Methods]
To prepare the osteoporosis model, ovariectomy (OVX) was performed using surgical methods. The prepared prescription [Shinkiwhan (SKH), a Korean medicine] was administered orally at a dose of 210 mg/kg/day for 8 weeks. After completion of the animal experiment, the bone mineral density (BMD) was analyzed using double-energy X-ray absorptiometry. The analysis of the effect of drugs on bones was performed using histological analysis and immunostaining.
Physical activity is effective in preventing and treating diverse diseases. However, westernized diets, sedentary lifestyles, and an irregular physical activity are increasing the incidence of disease in modern people. In particular, older people and postmenopausal women have an increased incidence of osteoporosis due to their inactive lifestyle. Osteoporosis is a representative disease in which bone dysfunction is characterized by a bone mass decrease and an abnormal bone microstructure [1]. According to the National Health & Nutritional Examination Survey, the prevalence of osteoporosis in the United States is estimated to be about 5 million people [2,3]. In particular, the prevalence rate among women who are over 50 years of age is rapidly increasing worldwide [4]. Therefore, it is urgent to diagnose osteoporosis, recognize the need for prevention and treatment, and find a treatment solution.
The skeletal system contains bones, ligaments, and cartilages that play multiple roles in the body, including the protection of internal organs and body structures and enabling movement [5]. Bones support the metabolic incorporation that serves to safely store essential minerals and blood cells [6]. Bones maintain their function through bone remodeling by continuously performing the roles of destroying and forming the bone, done by osteoclasts and osteoblasts, respectively [7]. Osteoprotegerin/receptor activator of nuclear factor kappa B/RANK ligand (OPG/RANK/RANKL) system is tightly involved in bone remodeling. RANK expresses bone activity, and RANKL promotes osteoclast differentiation [8,9]. Additionally, OPG plays a role in inhibiting osteoclast differentiation in the RANKL signaling system [10]. Hence, the analysis of changes in the OPG/RANK/RANK system also provides important clues for discovering osteoporosis treatment candidates.
The most useful method of osteoporosis diagnosis is by measuring the density of the lumbar spine and femur using double-energy X-ray absorptiometry (DXA) [11]. After diagnosis, the therapeutic option is to increase bone mineral density and decrease the frequency of fractures, if sufficient calcium is consumed. In addition, vitamin D intake is not only necessary for calcium absorption in the intestine, but also plays an important role in maintaining bone and muscle function and body balance. In pharmacological therapy, drugs that act as estrogen agonists or antagonists (raloxifene and bazedoxifene) and powerful bone resorption inhibitors (alendronate, risedronate, ibandronate, and zoledronate) are approved and used [12-17].
Natural products, such as plants, animals, microorganisms, and those of metabolic products may contain compounds that exert pharmacological activities that inhibit bone disease [18]. In Korea, there are many candidate substances that can make bones healthy in traditional oriental medicine. Osteoporosis requires a long-term treatment, and we believe that research to find natural products that help in osteoporosis treatment is necessary. However, oriental medical research has not yet been fully established. In the present study, therefore, we investigated the anti-osteoporosis effect of the natural product Shinkiwhan on osteoporosis in an ovariectomized mouse model. Our findings provide basic information for osteoporosis treatment.
Shinkiwhan, a Korean medicine, was obtained from Jungwoo medicines (Chungcheonnam-do, Korea). Rehmannia glutinosa Liboschitz ex Steudel (15 g), Dioscorea batatas Decaisne (7.5 g), Cornus officinalis Siebold et Zuccarini (7.5 g), Schisandra chinensis (Turcz.) Baillon (7.5 g), Alisma orientale Juzepzuk (6 g), Paeonia suffruticosa Andrews (6 g), and Poria cocos Wolf (6 g) were boiled in 2,000 mL of distilled water at 100°C for 3 h, and then, filtered. The decoction was reduced to 50 mL using a rotary evaporator. To obtain an extract of SKH, the supernatant was lyophilized at −60°C.
Experimental animals were 7-week-old female Sprague-Dawley rats (Sam Taco, Gyeonggi-do, Korea), which were used for experiments after acclimatization for 1 week. All experiments and animal care were performed in accordance with institutional guidelines (SEMCARE 16-06-01). Rats were divided into three groups (normal control: CON; negative control: OVXT; treatment group; SKHT). The CON was sutured without ovarian resection after laparotomy. The osteoporosis animal model was prepared using a surgical method. After resection of the rat ovaries, OVXT and SKHT were administered normal saline or a sample solution (a dose of 210 mg/kg/day) for 8 weeks. Experimental animals were kept in a standard cage in a breeding room maintained at a constant temperature of 25 ± 2°C, a humidity of 55 ± 5%, and 12- hour light/dark cycles, with standard feed containing 1.2% calcium and 0.8% phosphorus (DAMOOLSCIENCE, Daejeon, Korea).
After 8 weeks of OVX, anesthesia was performed using diethyl ether, and the femur was removed. The muscles attached to the femur were clearly removed and fixed in 10% neutral buffered formalin at room temperature for 24 h, followed by analysis. Bone density was analyzed using DXA (InAlyzer, Medikors, Seoul, Korea).
The bones were decalcified using a decalcification solution (Sigma, ST, MO, USA) for 12 h. The bones were washed and embedded in paraffin. The embedded bone samples were sectioned in 5 μm-thick slices. The sections were washed using a xylene solution and dehydrated using serial concentrations of ethanol (100%, 90%, 80%, 70%, and 60%). To observe the bone matrix, the sections were stained with 0.1% Safranin O solution for 5 min. Images were captured using an inverted microscope (Nikon, Tokyo, Japan).
Immunohistochemistry was performed as previously described [19]. To detect the expression of specific proteins, such as the phosphorylation of JNK, RANKL, and OPG, the sections were stained with specific antibodies. The 5 μm-thick sections were treated with proteinase K (20 μg/mL) for 5 min and incubated with 10% normal goat serum for 4 h. The sections were incubated with antibodies, such as anti-pJNK, anti-RANKL, and anti-OPG (Santacurz, TX, USA) at 4°C overnight. The sections were then incubated with the biotinylated secondary antibodies. Images were captured using an inverted microscope (Nikon, Tokyo, Japan). The photographs were analyzed using ImageJ software.
GC/MS analysis was performed as previously described [20], using an Agilent 6890N GC/5975i MS instrument (Palo Alto, CA, USA) and a DB5-MS capillary column (30 m × 250 μm, 0.25 μm film thickness). Helium was used as the carrier gas at a flow rate of 1 mL/min. The injector port and interface temperatures were 280°C and 300°C, respectively. The gas chromatography oven was maintained at 40°C for 2 min, increased to 230°C at a rate of 5°C/min, and then kept constant at 300°C for 5 min. The split ratio was 1:10, and the mass range used was 40 - 800 m/z.
Results are expressed as mean ± standard deviation (n = 6). Multiple comparisons were performed using oneway analysis of variance, followed by Tukey’s post-hoc test (GraphPad Prism ver. 4.00 for Windows, GraphPad, CA, USA). p-values < 0.05 were considered statistically significant.
The bone density of rats of each experimental group was measured using DXA. As shown in Figure 1, it was 0.2557 ± 0.0026 g/cm2 in CON, 0.1833 ± 0.0028 g/cm2 in OVXT, and 0.2196 ± 0.003 g/cm2 in SKHT.
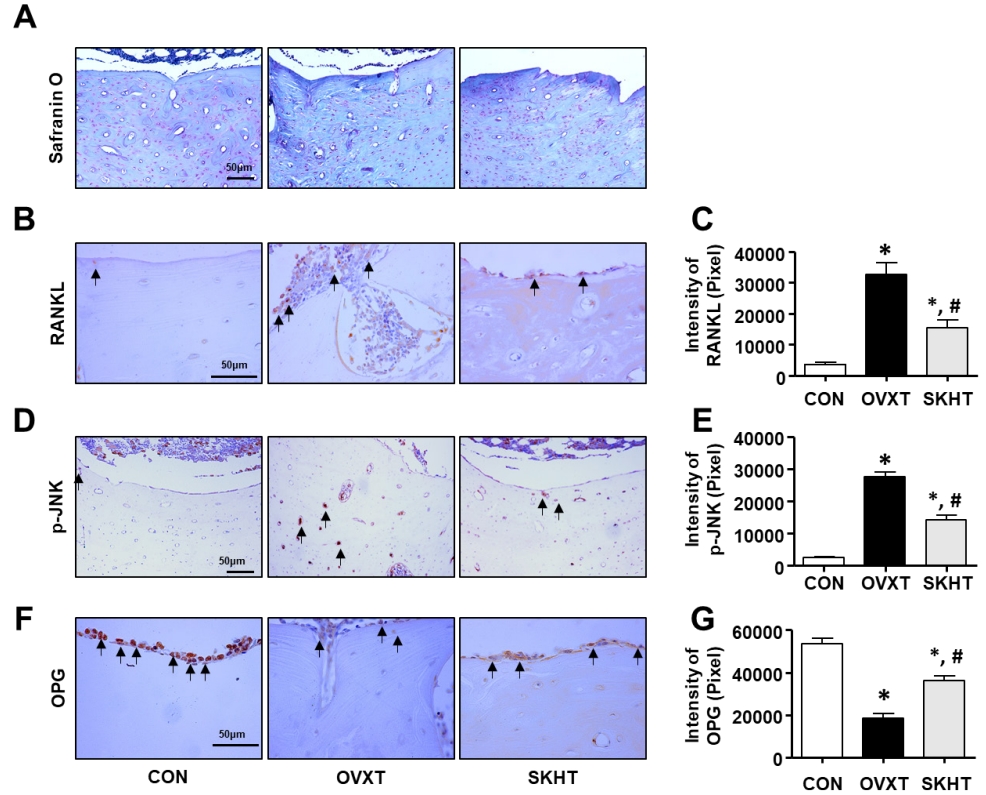
The distribution of glycosaminoglycan (GAG), a component of the bone matrix, was confirmed using safranin-O-fast green staining. As shown in Figure 2A, in the OVXT group, the distribution of positive GAG in the spongy and compact bones around the bone marrow decreased. In contrast, an increase in the distribution of GAG was observed in the SKHT group.
To investigate whether SKH can regulate the activity of osteoclasts, we performed immunohistochemistry assays with specific antibodies, such as anti-RANKL, anti-p-JNK, and anti-OPG. As shown in Figures 2B and C, the RANKL-positive response in the spongy bone of OVXT group was 33,551 ± 197/20,000,000 pixels, which was significantly increased, by 795%, compared to that of the CON group. The level of RANKL in the SKHT group was 15,280 ± 816/20,000,000 pixels, which was significantly decreased, by 53%, compared to that of the OVXT group. Next, as shown in Figures 2D and E, the p-JNK-positive response in the lamella of the compact bone of OVXT group (27,601 ± 478/20,000,000 pixel cells) increased by 1,205% compared to that of the CON group (2,497 ± 100/20,000,000 pixel cells). In the SKHT group, the positive p-JNK reactions (14,130 ± 481/20,000,000 pixel cells) showed a 48% decrease compared to that of the OVXT group. Next, as shown in Figures 2F and G, the OPG-positive response in the spongy bone of the OVXT group was 18,392 ± 674/20,000,000 pixels, which was significantly reduced, by 674%, compared to the CON group. The level of OPN in the SKHT group was 36,055 ± 713/20,000,000 pixels, which was significantly increased, by 96%, compared to the OVXT group.
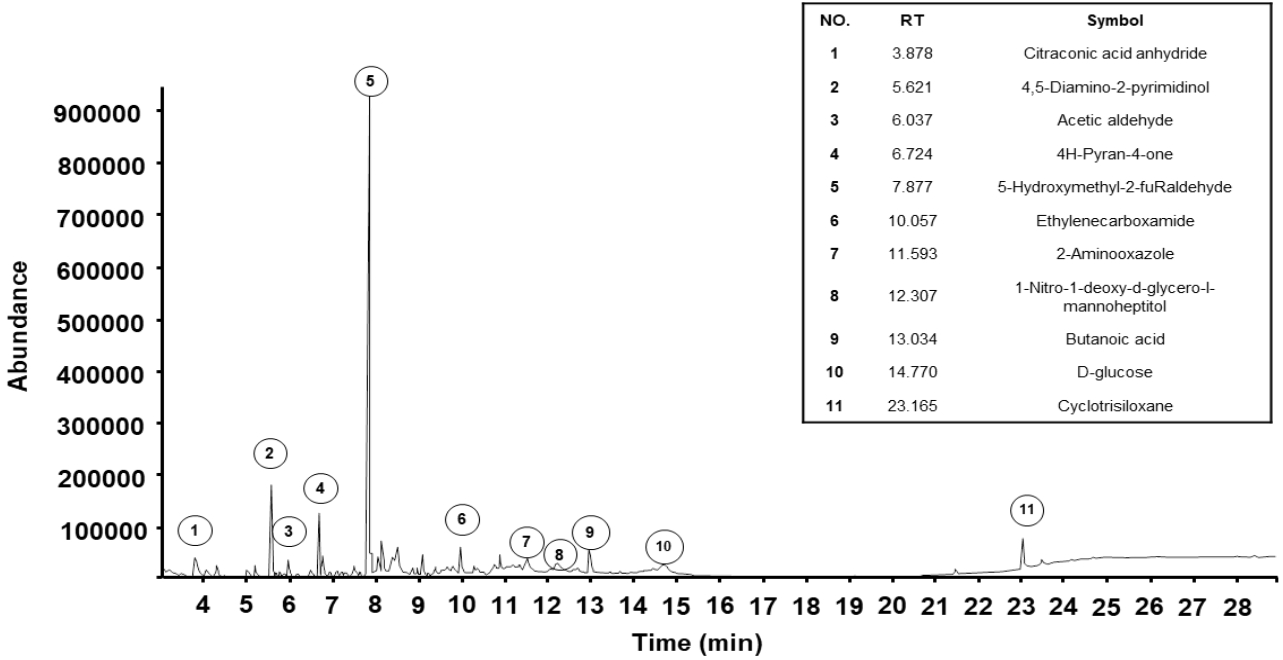
Component identification was performed using gas chromatography and mass spectrometry to analyze the compounds present in SKH. A total of 11 constituents of SKH were detected, as shown in Figure 3. The components included citraconic acid anhydride (5.70%), 4,5-Diamino-2-pyrimidinol (11.51%), acetaldehyde (3.63%), 4H-Pyran-4-one (6.01%), 5-Hydroxyl-2-furaldehyde (47.68%), ethylenecarboxamide (3.72%), 2-Amino-oxazole (4.18%), 1-Nitro-1-deoxy-d-glycero-l-mannoheptitol (2.43%), butanoic acid (4.50%), D-glucose (3.63%), and cyclotrisiloxane (5.27%).
In the present study, we evaluated whether a Korean medicine, SKH, can regulate osteoporosis in ovariectomized rats. Hence, we found that SKH had an inhibitory effect on bone mass loss in ovariectomized rats. The result was confirmed using a dual-energy X-ray absorptiometry (DXA) test. For the diagnosis of osteoporosis, a global reference standard is established by examining the BMD through X-rays of DAX. The normal value of BMD is within 1% of the standard deviation; if it is higher than 1.0% and lower than 2.5%, osteopenia is diagnosed; and if it is higher than 2.5%, osteoporosis is diagnosed [21]. In this study, the OVX model reduced the BMD by 25% compared to the normal group, whereas SKH intake showed a 15% reduction. Therefore, we suggest that SKH is effective against osteoporosis.
Bone is a physiologically active tissue that is repeatedly remodeled and regulated throughout life by osteoclasts and osteoblasts [4]. In particular, the imbalance of bone resorption and remodeling that causes osteoporosis can be caused by drugs, hereditary diseases, calcium imbalances, endocrine system abnormalities, digestive problems, and other diseases [22]. Osteoclasts play a role in bone resorption in small damaged areas of the bone [23]. In this process, RANKL expression increases through mitogen-activated protein kinase, such as phosphorylation of c-jun Nterminal kinases (JNK), and OPG performs the function of inhibiting RANKL [24]. Therefore, we suggest that SKH regulates osteoclast activity. However, these results have raised other questions about the effectiveness of SKH, including how it modulates the bone remodeling signaling pathway.
To address this question, we explored the profiles of the components of SKH using GC/MS. Our data showed that 5-hydroxyl-2-furaldehyde is a component of SKH. Tan et al. demonstrated that 5-hydroxyl-2-furaldehyde regulated osteogenic differentiation of bone mesenchymal stem cells [25]. Therefore, we suggest that SKH regulates bone remodeling through osteoblast differentiation.
Exercise has a good effect on maintaining overall vitality, such as heart circulation and psychological function. In particular, active people have a 50% reduction of the risk of osteophytes [26]. According to sports science reports, it is known that aerobics and weightlifting are effective in increasing the BMD of the spine or osteophytes [27]. M Y Chien et al. recommended an exercise protocol for bone remodeling that consists of, at least, 3 exercise sessions per week with an intensity above 70% of the maximal oxygen consumption for 30 min [28]. The molecular pathways of bone remodeling regulated by exercise have been studied and exercise-induced estrogen has been reported to inhibit bone resorption by regulating the expression of RANKL, TRPV5, and OPG [29]. However, patients with osteoporosis need to control the intensity of exercise. Osteoporosis is a disease that increases the risk of fracture due to a decreased bone density and microstructure damage in the bone tissue [30]. Therefore, we suggest that treating and preventing osteoporosis requires the intake of nutrients for bone regeneration and the use of effective health-functional substances. In addition, our further study should confirm the synergistic effect of SKH administration and exercise on bone remodeling.
In conclusion, our findings demonstrate that SKH regulates the expression of OPG, p-JNK, and RANKL in the bones of OVX-induced osteoporosis. In addition, SKH regulates BMD in OVX rats. These results suggest that 5-hydroxyl-2-furaldehyde and the other active compounds in SKH may have protective effects against osteoporosis.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF2019R1F1A105841412).
Figure 1.
The therapeutic effect of Shinkiwhan on ovariectomy-induced rat bone loss.
The bone mass was analyzed using dual energy X-ray absorptiometry (DXA).
(A) Representative DXA images of the femur of the control (CON) group, ovariectomized (OVXT) group, and the group of OVX rats treated with 210 mg/kg/day Shinkiwhan (SKHT). (B) The bar graphs show the bone density. Data are expressed as mean ± standard deviation. * P < 0.05 versus CON group. # P < 0.05 versus OVX group.
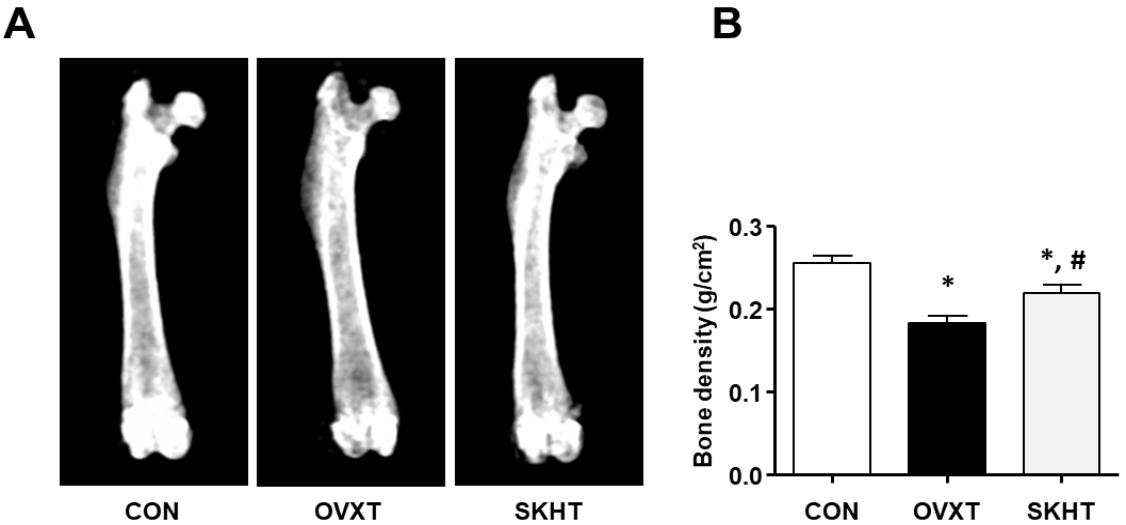
Figure 2.
The effect of Shinkiwhan in osteoclast activity of ovariectomized rats.
The rats were sacrificed and their femurs were isolated. The femurs were sectioned in 5-μm- thick slices. (A) Femurs were stained with Safranin O solution. (B, D, and F) The sections were stained with specific antibodies, such as phosphorylation of JNK (p-JNK), receptor activator of NF-kB ligand (RANKL), and osteoprotegerin (OPG). The positive signal reacts with 3,3'- diaminobenzidine and acquires a brown color. The block arrows indicate p-JNK, RANKL, and OPG positivity. (C, E, and G) The bar graphs indicate the brown intensity of the photographs in panels (B), (D), and (F), respectively. Data are expressed as mean ± standard deviation. *P < 0.05 versus CON group. # P < 0.05 versus OVX group.
REFERENCES
1. Chen H, Kubo KY. Bone three-dimensional microstructural features of the common osteoporotic fracture sites. World J Orthop 2014;5:86-95.

2. Looker AC, Orwoll ES, Johnston CC Jr, Lindsay RL, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res 1997;12:1761-8.


3. MacLean C, Newberry S, Maglione M, McMahon M, Ranganath V, Suttorp M, Mojica W, Timmer M, Alexander A, McNamara M, Desai SB, Zhou A, Chen S, Carter J, Tringale C, Valentine D, Johnsen B, Grossman J. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med 2008;148:197-213.


4. Datta HK, Ng WF, Walker JA, Tuck SP, Varanasi SS. The cell biology of bone metabolism. J Clin Pathol 2008;61:577-87.


6. Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood 2005;105:2631-9.



7. Baig MA, Bacha D. Histology, Bone. StatPearls [Internet] 2020.
8. Ominsky MS, Li X, Asuncion FJ, Barrero M, Warmington KS, Dwyer D, Stolina M, Geng Z, Grisanti M, Tan HL, Corbin T, McCabe J, Simonet WS, Ke HZ, Kostenuik PJ. RANKL inhibition with osteoprotegerin increases bone strength by improving cortical and trabecular bone architecture in ovariectomized rats. J Bone Miner Res 2008;23:672-82.


9. Yuan Y, Chen X, Zhang L, Wu J, Guo J, Zou D, Chen B, Sun Z, Shen C, Zou J. The roles of exercise in bone remodeling and in prevention and treatment of osteoporosis. Prog Biophys Mol Biol 2016;122:122-30.


12. Francis RM, Aspray TJ, Bowring CE, Fraser WD, Gittoes NJ, Javaid MK, Macdonald HM, Patel S, Selby PL, Tanna N. National Osteoporosis Society practical clinical guideline on vitamin D and bone health. Maturitas 2015;80:119-21.


13. Austin M, Yang YC, Vittinghoff E, Adami S, Boonen S, Bauer DC, Bianchi G, Bolognese MA, Christiansen C, Eastell R, Grauer A, Hawkins F, Kendler DL, Oliveri B, McClung MR, Reid IR, Siris ES, Zanchetta J, Zerbini CA, Libanati C, Cummings SR; FREEDOM Trial. Relationship between bone mineral density changes with denosumab treatment and risk reduction for vertebral and nonvertebral fractures. J Bone Miner Res 2012;27:687-93.



14. Bilezikian JP, Rubin MR, Finkelstein JS. Parathyroid hormone as an anabolic therapy for women and men. J Endocrinol Invest 2005;28:41-9.

15. Miller PD, Chines AA, Christiansen C, Hoeck HC, Kendler DL, Lewiecki EM, Woodson G, Levine AB, Constantine G, Delmas PD. Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-yr results of a randomized, double- blind, placebo-, and active-controlled study. J Bone Miner Res 2008;23:525-35.


16. Rozenberg S, Bruyère O, Bergmann P, Cavalier E, Gielen E, Goemaere S, Kaufman JM, Lapauw B, Laurent MR, De Schepper J, Body JJ. How to manage osteoporosis before the age of 50. Maturitas 2020;138:14-25.


17. Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, McNabb MA, Wenger NK. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 2006;355:125-37.


18. He J, Li X, Wang Z, Bennett S, Chen K, Xiao Z, Zhan J, Chen S, Hou Y, Chen J, Wang S, Xu J, Lin D. Therapeutic anabolic and anticatabolic Benefits of natural Chinese medicines for the treatment of osteoporosis. Front Pharmacol 2019;10:1344.



19. Park KS, Ahn SH, Lee KP, Park SY, Cheon JH, Choi JY, Kim K. The natural compound dansameum reduces foam cell formation by downregulating CD36 and peroxisome proliferatoractivated receptor-gamma; expression. Pharmacogn Mag 2018 J;13(Suppl4):S868-74.
20. Lee KP, Choi NH, Kim HS, Ahn S, Park IS, Lee DW. Anti-neuroinflammatory effects of ethanolic extract of black chokeberry (Aronia melanocapa L.) in lipopolysaccharide-stimulated BV2 cells and ICR mice. Nutr Res Pract 2018;12:13-9.



21. Martin RM, Correa PH. Bone quality and osteoporosis therapy. Arq Bras Endocrinol Metabol 2010;54:186-99.



22. Office of the Surgeon General (US). Bone health and osteoporosis: a report of the surgeon general. Rockville (MD): Office of the Surgeon General (US). 2004.
23. Morel A, Blangy A, Vives V. Methods to investigate the role of Rho GTPases in osteoclast function. Methods Mol Biol 2018;1821:219-33.


24. Ikeda F, Matsubara T, Tsurukai T, Hata K, Nishimura R, Yoneda T. JNK/c-Jun signaling mediates an anti-apoptotic effect of RANKL in osteoclasts. J Bone Miner Res 2008;23:907-14.


25. Tan XL, Zhang YH, Cai JP, Zhu LH, Ge WJ, Zhang X. 5-(Hydroxymethyl)-2-furaldehyde inhibits adipogenic and enhances osteogenic differentiation of rat bone mesenchymal stem cells. Nat Prod Commun 2014;9:529-32.


26. Tobeiha M, Moghadasian MH, Amin N, Jafarnejad S. RANKL/RANK/OPG pathway: a mechanism involved in exercise-induced bone remodeling. Biomed Res Int 2020;2020:6910312.




27. Benedetti MG, Furlini G, Zati A, Letizia Mauro G. The Effectiveness of Physical Exercise on Bone Density in Osteoporotic Patients. Biomed Res Int 2018;2018:4840531.




28. Chien MY, Wu YT, Hsu AT, Yang RS, Lai JS. Efficacy of a 24-week aerobic exercise program for osteopenic postmenopausal women. Calcif Tissue Int 2000;67:443-8.



-
METRICS

-
- 2 Crossref
- Scopus
- 3,953 View
- 37 Download
- Related articles in Phys Act Nutr