INTRODUCTION
Attention-deficit or/and hyperactivity disorder (ADHD) has been known to usually evoke hyperactivity, impulsiveness, and aggression, and the incidence of ADHD higher in boys than in girls1. ADHD occurs in school-aged boys and girls, and is characterized by difficulty in social relationships and lower study achievement2, 3. In a previous report, Korean children showed a higher ADHD incidence (3-20%)4 than children in the United States (3-8%)5 and Japan (7-12%)6.
The physiological mechanism of ADHD shows that lower activity levels of dopamine D4 receptor gene and norepinephrine mainly induce functional reduction in the right and dorsal frontal lobe of the brain7, 8. The brain-derived neurotrophic factor (BDNF) plays a key role in nerve cell division of dopamine9. Moreover, dopamine is involved in the regulation of the posterior attention system (PAS) related to action, memory, judgment, plan, and response. Meanwhile, norepinephrine is involved in the regulation of the anterior attention system (AAS) related to stimulus selection and choice, and attention distribution, maintenance, and change. Accordingly, malfunction of PAS and AAS may cause severe problems in execution functions such as personal relationships and coordination10, 11. Malfunction in PAS and AAS activates slow brainwaves via quantitative electroencephalography (QEEG). Therefore, activating the theta-to-beta wave ratio would be useful to improve physiological symptoms in children with ADHD12, 13, 14. In addition, children with ADHD need to have medical treatment and management as early as possible because only 20% of these children recover as they age and most continue to have ADHD through their adulthood8.
Methylphenidate and amphetamine are drugs used for the treatment of ADHD; however, approximately 20% of these medicines have been reported to evoke side effects such as hypertension, sleep disorder, and mood disorder, and just temporarily reduce ADHD symptoms15, 16. Thus, exercise therapy for children with ADHD has been suggested to activate brain function17. A previous study reported that exercise therapy and/or physical activity combined with cognitive function training for children with ADHD positively affected ADHD18. Horseback riding or hippotherapy has been a useful therapy for enhancing neuromuscular function since the 1970s in Europe and the United States19. Use of animals such as horses in therapy would be effective for positively changing psychological factors, social positioning act, quality of life, and movement function in children with ADHD20, 21. Horseback riding has been known to be effective as a high-intensity physical activity (>4 METs)22. Thus, hippotherapy would apply to functionally improving behavioral modification and psychological factors in children with ADHD. In addition, previous studies confirmed that above-moderate-intensity hippotherapy was positively effective for functional improvement in children with ADHD17, 23.
EEG neurofeedback training has been defined as a comprehensive training system by changing the brain function of children with ADHD24 and can generally be applied as a treatment method for enhancing brain function in children with ADHD because it is programmed for brain function improvement12, 25. Recent Internet technology uses a server-based long-distance learning system that enables patients to easily use the EEG neurofeedback training program and maximizes patientsŌĆÖ participation at home. In addition, this would be practically applicable for better therapeutic results in children with ADHD. Therefore, the purpose of this study was to investigate the combined effect of hippotherapy and EEG neurofeedback on brain function and blood BDNF level in children with ADHD.
METHODS
Participants
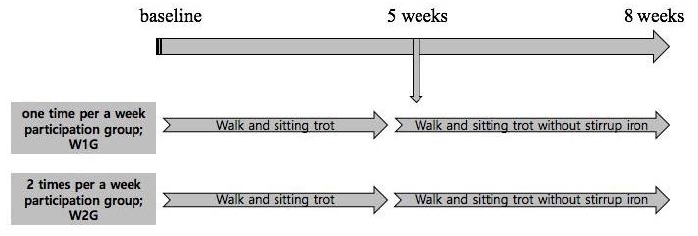
The study participants were patients with ADHD who were recruited from the pediatric psychiatry departments of G university and C university hospitals. Parents, physicians, and children with ADHD understood the study purpose and signed the study consent form. Previous medical history information was collected, and a psychological medical examination was conducted for calculating the attention quotient (AQ) for selecting the study patients. To examine the changes in serum BDNF level and brain function in children with ADHD followed by the primary training, 16 participants were divided into the ŌĆ£one time per weekŌĆØ group (W1G, n = 8) and the ŌĆ£two times per weekŌĆØ group (W2G, n = 8) during the 8-week training period. The secondary training was designed for W1G and combined with neurofeedback training, with 7 children with ADHD as participants (Table 1).
Hippotherapy program
Primary training
Primary training was conducted to investigate the changes in serum BDNF level and brain function in the children with ADHD, followed by the 1- and 2-time hippotherapy programs during the 8-week training period. The participants in W1G and W2G performed the walk and sitting trot training during the first 4 weeks and the walk and sitting trot training without a stirrup iron after 5 weeks of training (Figure 1).
Secondary training
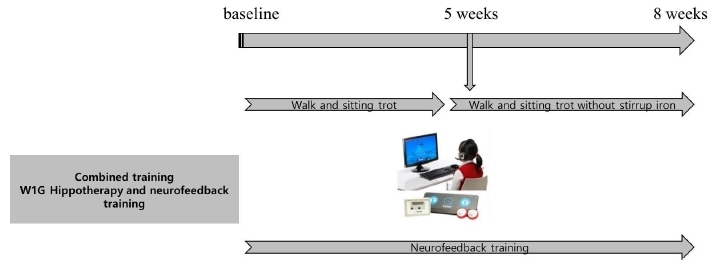
In W1G, training was combined with neurofeedback training. To examine the changes in serum BDNF level and brain function, the prefrontal lobe of the brain was trained using the NH neurofeedback system through the sequential bipolar montage for 30 minutes. The brain wave range was set at 45 Hz (Figure 2).
Blood BDNF and functional magnetic resonance imaging analysis
Blood BDNF analysis
Blood BDNF level was measured at baseline and after the hippotherapy and combination trainings. Blood BDNF level was analyzed using human enzyme-linked immunosorbent assay kits (AB Frontier). Blood was collected from the forearm venous vein, and the collected blood was stored in serum tubes. It was centrifuged at 1000├Śg (relative centrifuged force) for 15 minutes, and 0.1 ŃÄ¢ of separated serum was analyzed using the Microplate model 680 (Bio-Rad, USA). Then, the blood sample was analyzed using a human BDNF kit, followed by the ELISA method. The BDNF level was calculated by measuring optical density.
Brain function test using functional magnetic resonance imaging
To measure brain function, functional magnetic resonance imaging (fMRI) was used. A 3.0-Tesla GE MR scanner (General Electric Medical Systems, Milwaukee, WI) was used to check the anatomical structure of the brain. T-1 imaging as the 3-D spoiled gradient echo pulse sequence (SPGR) was also used to scan 240 sagittal images of the brain. A T-2 image scan was used to check the brain structure. A fMRI scan was conducted using the quadrature-type head coil, and MR sequence was shown (Table 2).
Table┬Ā2.
Magnetic resonance (MR) sequence.
EEG and comprehensive attention test followed by the combined training
EEG (electroencephalogram) test
EEG was used to test brain waves and Neuro H. Neurofeedback System was used. Neuro H. System is a portable 2 Channel System digital brain wave measuring instrument and it measures the both frontal robes by using the Sequential Bipolar Montage. Brain Quotient (BQ) program was used to test brain waves.
Comprehensive attention test measurement
Comprehensive attention test (CAT) is a standardized attention test that includes 6 subcategories, namely visual simple selection attention capacity, auditory simple selection attention capacity, suppression continuous attention capacity, interference selection attention capacity, division attention capacity, and job memory capacity. CAT is a computer-based program that takes 30-40 minutes to complete and calculates the testerŌĆÖs score. CAT can report emotion and behavioral scores of children with ADHD. This measurement was used only in the secondary training.
Data Analysis
Descriptive statistics were calculated using the means and the standard deviations from the collected data. The two-way mixed analysis of variance (group ├Ś repetition) was used to test the changes in serum BDNF level after the primary training. In addition, to examine the fMRI results for brain function changes, a paired-sample T test was conducted by converting image data into numeric data and to compare the difference between the baseline and after the 8-week training. To confirm the efficacy of the secondary training, a paired-sample T test was conducted. The significance level in all the tests was set at p < 0.05.
RESULTS
Primary training
BDNF level
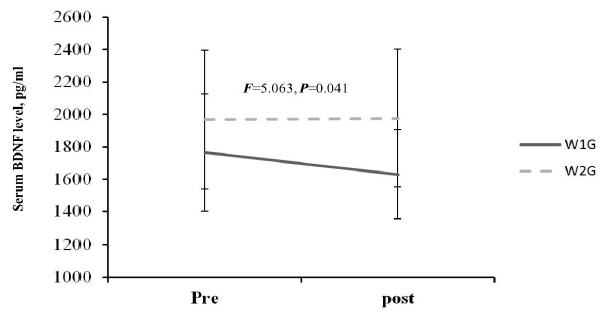
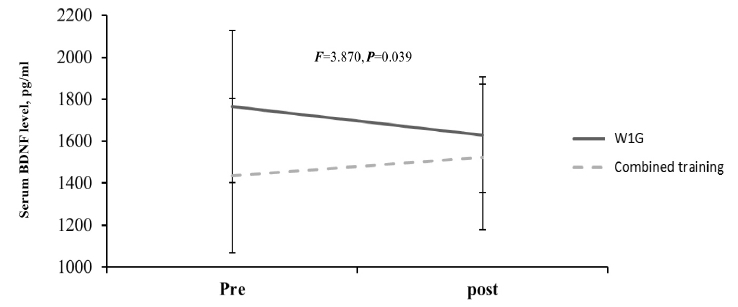
The blood BDNF level change after hippotherapy based on hippotherapy frequency showed a decreased tendency in W1G from before (1766.03 ┬▒ 362.54 pg/ml) to after training (1630.65 ┬▒ 276.70 pg/ml). By contrast, the blood BDNF level in W2G showed an increased tendency from before (1968.28 ┬▒ 429.08 pg/ml) to after training (1976.28 ┬▒ 425.35 pg/ml), which showed a significant interaction (F = 5.063, p = 0.041; Figure 3.). Considering the mean difference between the groups at baseline, delta differences (pre-post) were compared, and delta was increased in W2G but was decreased in W1G, with a significant difference between the groups (p < 0.05). Moreover, W1G combined with neurofeedback training showed an increased blood BDNF level from before (1436.57 ┬▒ 368.76 pg/ml) to after (1525.23 ┬▒ 346.22 pg/ml), which showed a significant group ├Ś repetition interaction (F = 3.870, p = 0.039; Figure 4).
Functional magnetic resonance imaging
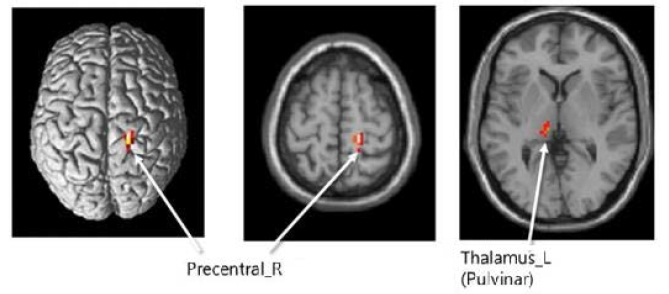
Brain activity changes after hippotherapy showed a decreased tendency in the right precentral cortex in W1G and W2G; otherwise, no significant difference was observed. On the contrary, the left thalamus activity in both groups showed a decreased tendency and a significant lower change in W2G than in W1G (p < 0.05; Table 3).
Table┬Ā3.
Brain function changes based on hippotherapy frequency
Secondary training
Combined effect of hippotherapy and neurofeedback training on BDNF level
As shown in Figure 4, the combined effect of hippotherapy and neurofeedback on BDNF level showed a decreased tendency in W1G (pretraining, 1766.03 ┬▒ 362.54 pg/ml; posttraining, 1630.65 ┬▒ 276.70 pg/ml). By contrast, the BDNF level in W2G showed an increased tendency (pretraining, 1968.28 ┬▒ 429.08 pg/ml; posttraining, 1976.28 ┬▒ 425.35 pg/ml). Moreover, combined training showed a significant group ├Ś repetition interaction in W1G (pretraining, 1436.57 ┬▒ 368.76 pg/ml; posttraining, 1525.23 ┬▒ 346.22 pg/ml; F=3.870, p = 0.039).
Combined effect of hippotherapy and neurofeedback training on brain function
Brain wave (BQ) changes after the 8-week combined training with hippotherapy and neurofeedback did not induce significant changes in basic rhythm (Left┬ĘRight), self-control index, attention index (Left┬ĘRight), activation index (Left┬ĘRight), emotion index, anti-stress index (Left┬ĘRight), left and right brain balance, and brain index. This might have resulted from taking medication during the 8-week training, and emotional expressions seem to be absent at the pretest (Table 4).
Table┬Ā4.
Combined training effect on brain wave changes (BQ)
As shown in Table 5, the left and right bilateral middle frontal cortex area and left precentral cortex showed an increased brain function activity after combined training.
Combined effect of hippotherapy and neurofeedback training on CAT score
As shown in Table 6, the combined effect of hippotherapy and neurofeedback training showed a significant decrease in false-alarm error of suppression continuous attention capacity test (t = 2.66, p = 0.04). An improved tendency of false-alarm error in the visual (t = 1.58, p = 0.17; t = 0.32, p = 0.76) and auditory simple selection attention capacity tests (t = 0.48, p = 0.65; t = 1.07, p = 0.33; t = 0.35, p = 0.74) were observed. However, a significant decrease in reaction time in the auditory simple selection attention capacity test (t = ŌłÆ2.66, p = 0.04) was found.
Table┬Ā6.
Comprehensive attention test changes after combined training
DISCUSSION
This study suggests that hippotherapy might induce neurotransmitter activation in children with ADHD because they usually have lower neurotransmitter activation7, 8 and are expected to have increased activities in the right and dorsal frontal lobes. This study proved that the left thalamus activity in W2G was significantly higher than that in W1G. However, participation twice per week in the hippotherapy program may practically cause a cost-effectiveness problem; thus, children with ADHD are recommended to participate once per week in the hippotherapy program combined with neurofeedback training because neurofeedback training has a comparably lower cost and is as fun and interesting as a game for children. In this study, we found a significant job memory increase of the left bilateral middle frontal cortex, right bilateral middle frontal cortex, and left precentral cortex after by hippotherapy and neurofeedback training. Moreover, this study confirmed that the decision-making area of the brain activity was increased during task performance.
This study also suggests that skeletal muscle improvement in children with ADHD throughout horseback riding might regulate histone deacetylase (HDAC) from skeletal muscles. McGee et al.26 also suggested that regular physical activity might induce HDAC regulation and thus blood BDNF activation. These study results showed that the blood BDNF level in W1G combined with neurofeedback training was significantly increased in comparison with that in W2G. This study result may have been induced by the HDAC regulation caused by the muscle contraction after hippotherapy participation and brain function changes at the cell level after neurofeedback training12, 24, 25.
Increased left thalamus activity in W2G may be effective in suppressing caloric intake and might be expected to be effective for reducing oversupplied caloric intake in children with ADHD. This study did not investigate the hyperactivity pattern in children with ADHD and may not clearly present behavioral pattern changes via the hippotherapy program combined with neurofeedback training. Nevertheless, the blood BDNF changes after combined training showed a possibility to contribute to behavioral pattern changes throughout the hippotherapy program combined with neurofeedback training. Mattson (2010)27 insisted on a negative association between dietary energy intake and blood BDNF level, which supports our finding. Our study results showed a significant increase in blood BDNF level after combined training, which may induce brain function improvement in children with ADHD. No previous study related to the present study has been conducted; thus, we cannot clearly compare our study results to previous study results. However, we can expect a positive combined effect of hippotherapy and neurofeedback training because this study found significant cerebral cortex activity changes. Moreover, the present study results showed no significant difference in serum BDNF level between the primary and secondary trainings. However, this would be considered as a crucial factor for understanding the overall study results. We can assume that the sensitivity of serum BDNF concentration was affected by the analytical method by removing plasma protein such as fibrinogen, which resulted in a decreased BDNF concentration and was affected by the muscle contraction in children with ADHD before blood drawing. In relation with this assumption, Cho et al.28 reported that muscle contraction could induce platelet activation and affect changes in serum BDNF level changes. To clarify this assumption, an adjusted method for examining serum BDNF level changes, followed by the physical activity level, in children with ADHD should be considered in future intervention studies.
This study has two limitations. First, the ADHD level of each participant might be inconsistent, which might have negatively affected the study results. Second, the progress of each participant in hippotherapy differed because of the different levels of learning ability. This might have caused a variation in exercise intensity, although the study set the same exercise intensity. We recommend that future studies consider consistency of ADHD level and unified exercise intensity application for better understanding of the effects of hippotherapy and neurofeedback training on children with ADHD.
CONCLUSION
Enhanced brain function was observed in the children with ADHD as their participation frequency in hippotherapy increased. However, a cost-effectiveness problem may be evoked as hippotherapy participation frequency is increased. Therefore, participation once per week in the hippotherapy program combined with neurofeedback training would optimize cost-effectiveness and brain function enhancement in comparison with participation twice per week in the hippotherapy program. This study suggests that hippotherapy combined with various psychological interventions would be useful for improving brain function in children with ADHD.