A study on verifying the effectiveness of 4-week composite weight-loss dietary supplement ingestion on body composition and blood lipid changes in middle-aged women
Article information
Abstract
Purpose
The purpose of this study was to investigate the effectiveness of a composite weight-loss dietary supplement on body composition and blood lipid changes in middle-aged women.
Methods
Thirty seven middle-aged women living in the Kyunggi area participated in this study and they were randomly divided into 2 groups (Dietary supplement ingestion group; DG, n = 20 and Placebo group; PG, n = 17). Blood draw and dual energy x-ray (DEXA) measurements were conducted to examine changes in body composition and blood lipids.
Results
There were no significant changes in weight and BMI in both groups. There was an interaction between the composite weight-loss dietary supplement intake and lean body mass in DG and there was a significant decrease in percent body fat in DG. Blood lipid changes in the study results showed that there was no significant difference in TC, TG, and LDL in both groups; however, there was a significant interaction between the composite weight-loss dietary supplement intake and HDL-C as well as an increase in the HDL-C of DG.
Conclusion
In conclusion, it seems that 4-week ingestion of the composite weight-loss dietary supplement decreased body fat, increased lean body mass, and increased HDL-C. Therefore, the composite weight-loss dietary supplement is expected to prevent obesity and induce health improvements in middle-aged women.
INTRODUCTION
It was recently reported that the incidence of lifestyle diseases is increasing due to lower levels of physical activity resulting from fast economic development and changes in dietary habits [1]. Especially, women over 50 years old are reported to have higher incidence of metabolic disorders including obesity due to aging and women in their 60 have double the risk of men at the same age [2]. Middle-aged women usually experience menopause. This causes physical changes such as dramatically decreased physical fitness and physical activity levels, leading to obesity. Obesity in middle-aged women may directly and/or indirectly affect diabetes, hypertension, and hyperlipidemia, which might negatively affect health in old age [3]. It seems that middle-aged women can successfully decrease their body weight and maintain an optimal body weight by reducing caloric intake and increasing physical activity. Several previous studies mentioned that active standard treatment methods such as caloric restriction (dietary treatment) and increased physical activity (exercise treatment) may produce the best results for a healthy life. However, it is not easy to maintain these standards for a long period of time [4]. In addition, middle-aged women usually have increased physical risk for climaterium and cancer as well as increased psychological and social anxiety for their children and economic issues. Therefore, health care for middle-aged women should be considered important for the rest of their lives in an aging society [5]. Due to these health problems in middle-aged women, the development of programs and research related to dietary treatment and/or exercise treatment for obesity have recently increased [6,7]. However, most previous studies were criticized for not recognizing the fundamental importance of improved dietary intake due to their simple treatment methods such as rapid dietary restriction in a short period of time and evaluating the effect of the dietary program based on body weight-loss [8].
It is not easy to maintain optimal body weight for most people and obesity has become one of the most common diseases in the world. Consequently, obesity specialists continuously search for easier methods to induce weight loss by conducting research on plant based food and plant derived drugs [9]. Thus, it is essential to develop healthy functional foods for obesity patients using natural products [10]. Ingestion of a composite weight-loss dietary supplement for 4 weeks while maintaining a normal diet has the potential to reduce the difficulties that contemporary people experience when trying to lose weight. The supplement is mainly composed of plant based ingredients including Hydroxycitiric acid (HCA), puer tea, lotus leaf extract, red ginseng, slendesta, aloe, and grain enzymes. HCA is extracted from Garsinia Canbogia and it directly affects fatty acid synthesis, lipogenesis, appetite, and weight control. Therefore, HCA has been combined with other ingredients since the 1990’s to produce functional dietary supplements for losing weight, protecting heart function, controlling lipids, and improving endurance [11]. Puer tea including green tea has been reported to have an anticarcinogenic effect and to prevent coronary artery disease in animal studies. It is a strong antioxidant capable of changing the total cholesterol and LDL-C concentration in the blood. Additionally, it has been reported that puer tea increases energy expenditure and affects weight loss [12,13]. Lotus leaf extract has been reported to have an anti-obesity function and to increase endocrine and lipid metabolism. It also affects blood glucose and lipids by decreasing total cholesterol, triglyceride, and fasting blood glucose in diabetes-induced animal models [14]. Slendesta, a protein extracted from potatoes, can reduce the feeling of hunger and is used for treating chronic diseases such as cancer, hypertension, atherosclerosis, heart diseases, and liver diseases [15]. This study examined the effects of composite weight-loss dietary supplement ingestion on blood lipids and body fat in middle-aged women interested in weight loss.
Therefore, the first purpose of this study was to investigate the effect of the composite weight-loss dietary supplement in middle-aged women who maintained a normal diet and consumed the supplement 3 times a day for 4 weeks, with the goal of providing an easy-to-follow weight loss treatment option. The second purpose of this study was to investigate the effect of the primary ingredients of the composite weight-loss dietary supplement including HCA, puer tea, lotus leaf extract, red ginseng, slendesta, and aloe and to offer a healthy weight loss program that maintains the nutritional balance and prevents lean mass loss.
METHODS
Subjects
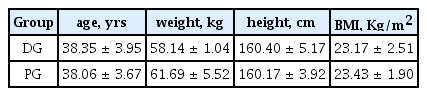
Thirty seven middle-aged women living in the Kyunggi area participated in this study and they were randomly divided into 2 groups (Dietary supplement ingestion group; DG, n = 20 and Placebo group; PG, n = 17). All subjects participated in the 4-week trial. Participants who did not meet the participant selection criterion for this study were excluded during screening. The participant selection criteria were as follows; 1) females aged from 35 to 50 years, 2) fasting blood glucose under 200mg/dl, and 3) no cardiovascular disease history. The characteristics of the participants are shown in Table 1.
Study design
This study utilized an open-label trail design over 4 weeks. The study purpose was fully explained to all participants and consent forms were obtained from all participants prior to the experiment. After the screening procedure, the selected participants were tested to obtain the baseline measurement and intake of the dietary supplement proceeded for 4 weeks. At the end of the 4-week trial, blood draw and dual energy X-ray absorptiometry (DEXA) measurements were conducted. After completing the trial, participants with drug compliance below 80% were excluded. The detailed study design is shown in Fig. 1.
Components of the composite weight-loss dietary supplement and the treatment method
Main components of dietary supplement and dose
Natural extracts were produced by the functional health food research center at the Aribio Company (Republic of Korea) and they were made into several forms such as drinks, tablets, powder, and capsules for convenience. During the experimental period, all participants received a monitoring call on the phone 3 times a day as a safety check. Placebo drugs for PG had the same package, color, taste, and flavor as the composite weight-loss dietary supplement.
Drinks
HCA, puer tea, and lotus leaf extract were consumed as drinks and the dosage was 230ml
Powder
Red ginseng concentrate and grain enzymes were in powder form and the dosage was 3g
Tablets
Slendesta extracted from potatoes and riboflavin were made into tablets and the dosage was 400mg
Capsules
Aloe, vitamins, and minerals were made into capsules and the dosage was 500mg
The composite weight-loss dietary supplement intake method
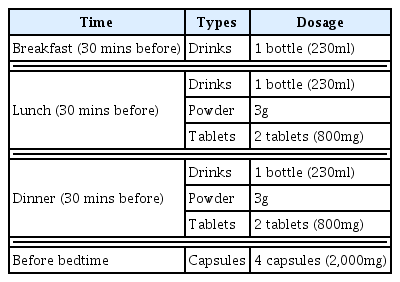
Intake of the composite weight-loss dietary supplement occurred 4 times a day (at 30 minutes before each meal and before bedtime) over 4 weeks. Meal size and the amount of physical activity (exercise program) were not controlled or restricted. The detailed intake method is presented in Table 2.
Measurement items and methods
Body fat measurement
DEXA (HV-PS 7681, Lunar, USA) was used to measure body fat changes. Metals such as necklaces and watches were removed and all participants wore patient gowns during DEXA scanning to prevent measurement errors. DEXA measurement was conducted twice (at baseline and after 4 weeks of dietary supplement intake).
Blood draw and analysis
Blood was drawn from the forearm vein of all participants at 06:00 after 12 hours of fasting. All blood samples were put into EDTA tubes and serum tubes and centrifuged (3000rpm: 15minutes) prior to analysis.
Total cholesterol
Total cholesterol was analyzed by enzyme colorimetry using a spectrophotometer. A quantified total cholesterol kit was used. A 20 μl sample was added to the 1,000 μl kit after 1-minute of preliminary heating. The mixture was kept in a water bath at 37°C for 5 minutes and then total cholesterol was measured.
Triglyceride
Triglyceride was analyzed by enzyme colorimetry using a spectrophotometer. A quantified triglyceride kit was used. A 20 μl sample was added to the 1,000 μl kit after 1-minute of preliminary heating. The mixture was kept in a water bath at 37°C for 5 minutes and then total cholesterol was measured.
HDL-C & LDL-C
High and low lipoprotein cholesterol analysis was performed by using 6ml vacutainer blood sample serum tubes containing SST gel and clotting activator. A Nedin (Korea) quantified lipoprotein cholesterol kit was used for evaluation at 505nm.
Data Analysis
SPSS 21.0 for Windows (Chicago, IL, USA) was used to process the study data and descriptive statistics was conducted. Two-way mixed ANOVA was used to test the time effect, interaction, and group differences between DG and PG. Statistical significance was set at p < .05.
RESULTS
Body fat changes
After completing the 4-week trial, there was no significant difference in weight and BMI in DG and PG. However, there was a greater significant interaction between lean body mass and dietary supplement treatment in DG compared to PG (p < .05). The significant decrease in percent body fat confirmed that the time effect was valid (p < .05) (Table 3).
Blood lipid changes
Blood lipid change results showed that there was no significant difference in TC, TG, and LDL in both groups. However, there was a significant interaction between the dietary supplement and HDL-C (p < .001) as well as an increase in HDL-C in DG (p < .001) (Table 4).
DISCUSSION
The composite weight-loss dietary supplement tested includes HCA, puer tea, lotus leaf extract, red ginseng, slendesta, and aloe. HCA is known to suppress lipid synthesis by impeding free fatty acid (FFA) synthesis, suppress appetite by stimulating the hypothalamus, and improve lipid oxidation by reducing Malonyl-CoA concentration [16]. Previous studies demonstrated that 12-week intake of HCA (1500mg/day) decreased body fat by promoting lipid oxidation in obese subjects [17]. Intake of slendesta and lotus leaf extract was reported to decrease BMI, percent body fat, and android and gynoid fat in female university students [10]. Similar to previous studies, the results of this study showed that HCA induced a decrease in body fat although HCA did not have a significant interaction with body fat changes (Fig. 2).
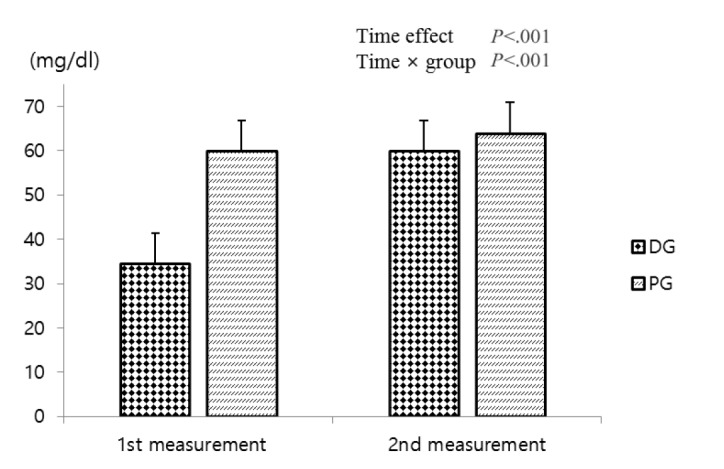
Puer tea is made of various chemical complexes including polyphenols, which have gained increasing attention for weight loss applications [18]. A previous study reported that epigallocatechin gallate (EGCG), one of the polyphenols, evoked quick weight loss for 2–7 days in rats. The rats returned to their original weights once EGCG administration ceased [19]. The present study demonstrated that HDL-C increased significantly increased in DG following dietary supplement ingestion (Fig. 3). Based on this finding, lotus leaf extract may promote lipid metabolism similar to previous study results. It also seems that alkaloids, gerbstoff, and the mucus components of lotus leaf extract induced positive effects on inflammation suppression and blood circulation improvement [20].
It has been reported that potato extract suppresses 3T3-L1 preadipocyte proliferation and decreases the leptin level from preadipocytes to insulin [21]. A previous study also found that aloesin from aloe might induce adiponectin revelation in adipocytes and thus, might control blood glucose and lipids [22]. Recently, it was reported that aloe itself and/or a certain complex molecule from aloe possesses a significant function in controlling blood glucose and lipids [23,24].
The study design did not restrict exercise and did not include an exercise program although the participants were encouraged to perform regular exercise at least 3 times per week. Based on the study results, lean body mass was increased in DG even though no exercise regimen was utilized. This was attributed to red ginseng. It has been reported that matol, one of the components of red ginseng, alleviates tissue damage in the body from free oxygen radicals and also decreases MDA (the end product of peroxidation lipids) concentration [25]. Free oxygen radicals usually come from oxidation during exercise and induce tissue damage in the body as well as cell and organ decline [26]. The human body has an antioxidant system for protecting itself from free oxygen radicals and it seems that red ginseng may help this process. In addition, red ginseng has been reported to save glycogen by maximizing FFA use, to increase exercise duration by suppressing protein catabolism, to promote glycogen synthesis after exercise, and to help maintain the level of creatine phosphate [27]. Although this study did not control dietary intake and physical activity, the results confirmed that 4-week dietary supplement ingestion with a normal diet has positive results such as increased lean body mass, decreased body fat, and increased HDL-C.
This study had several limitations. First, the total calorie intake for all participants was not collected and analyzed during the treatment period. Second, exercise intervention was not conducted. Lastly, the 4-week treatment period was used for practical reasons, but might be too short to determine the exact effects of the dietary supplement.
CONCLUSIONS
This study confirmed that 4-week dietary supplement ingestion positively affects lean body mass maintenance, body fat loss, and HDL-C in middle-aged women. Further studies need to consider an association between dietary supplement trial and aerobic exercise intervention. This is expected to produce a clearer effect for the dietary supplement.