The interaction effects of aerobic exercise training and vitamin D supplementation on plasma lipid profiles and insulin resistance in ovariectomized rats
Article information
Abstract
Purpose
The purpose of this study was to determine the interaction effects of aerobic exercise training and vitamin D supplementation on indices of obesity and plasma lipid profiles in ovariectomized (OVX) rats.
Methods
Forty female Wistar rats were divided into 5 groups: aerobic training (3 days/week for 8 weeks; AT; n = 8), aerobic training and vitamin D supplementation (OVX + AT + Vit D; n = 8), vitamin D supplementation (OVX + Vit D; n = 8), ovariectomized control (OVX + C, n = 8) and SHAM (n = 8). After blood sampling, visceral fat was taken from the abdominal cavity and weighed immediately. Data was statistically analyzed by One-way ANOVA and Repeated measure ANOVA tests with a 0.05 significance level.
Results
Body weight, visceral fat, BMI and food intake decreased significantly in OVX + AT + Vit D (P < 0.001); whereas these variables increased significantly in OVX + C (P < 0.001) and SHAM (P < 0.023) groups. At the end of two-months of follow-up, we observed significant differences in TC, TG, HDL-C, LDL-C, glucose, insulin, and HOMA-IR in all groups.
Conclusion
It seems that aerobic training with vitamin D, due to the involvement of muscle mass and exposure to dynamic pressure on the bones and muscles, increased energy expenditure, stimulated insulin exudation and glucose homeostasis, decreased insulin resistance and improved the lipid profile in ovariectomized rats.
INTRODUCTION
Obesity as a common problem in menopausal women is a major risk factor for the development of cardiovascular diseases. This is due to the importance of estrogen in the determination of body fat distribution [1,2]. Previous studies showed that deficiency of this hormone leads to an accumulation of visceral fat [3,4], and consequently, a decrease in insulin sensitivity [5,6]. Obesity is often associated with cardiovascular disease risk factors such as hypertension, dyslipoproteinemia, diabetes, and elevated inflammation markers [7–9]. Accumulating evidence suggests that altered vitamin D homeostasis may also contribute to an increased cardiovascular disease risk in obese subjects. There are positive correlations between low 25-hydroxyvitamin D [25(OH)D; < 43 nmol/L] and obesity [10] as well as between 25(OH)D < 33–37.5 nmol/L and cardiovascular mortality [11]. Vitamin D has a protective effect on bones [12], anticancer [13] and anti inflammatory [14] effects and its receptors are present in pancreatic b cells and insulin sensitive tissues including skeletal muscle tissues [15]. Vitamin D repletion improves insulin and glucose homeostasis in animal models of vitamin D deficiency [16,17]. It has also been reported that vitamin D deficiency in the blood is greatly associated with various metabolic factors related to diabetes such as hyperglycemia and insulin resistance [18,19], but data supporting this relationship are sparse. Furthermore, the role of vitamin D in adiposity remains unclear.
There are some studies reporting physical activity as an important environmental factor associated with body weight regulation and increasing physical activity has become an important aspect of non-pharmacological strategies to control obesity, weight gain and blood glucose in addition to preventing progression from impaired glucose tolerance [20,21]. Due to this connection, the American Diabetes Association (ADA) recommends aerobic exercise of medium intensity such as 150 minutes of walking per week, or 75 minutes of high intensity aerobic exercise per week for patients with type 2 diabetes [22]. Previous studies showed that only 28% of postmenopausal women perform regular physical activity [23,24]. Despite the beneficial effects of physical activity, there are many complications to achieving the predicted improvement in health. First of all, menopausal women are predisposed to osteoporosis and tend to avoid involvement in different kinds of physical activity. Second, it was recently reported that exercise is not that effective in some obese people [25,26]. The mechanisms by which the central nervous system or hormones interact with metabolic pathways and restrict weight loss are not yet understood. Here, we assumed that vitamin D could be one of the determining factors in achieving the health benefits of exercise. Therefore, the authors of this paper intended to investigate the effects of implementing vitamin D intake for 8 weeks in addition to aerobic exercise training on the plasma lipid profiles and insulin resistance in ovariectomized rats.
METHODS
Animal care
Forthy female Wistar rats weighing 240–255 g were housed four per cage and fed standard-pellet rat chow and tap water ad libitum. The 12-h:12-h light cycle (in which all fluorescent lighting was shielded, preventing the endogenous production of vitamin D3) began at 07.00 and the room temperature was maintained at 21–24°C. The experiments described in this study were conducted according to the policy of the Iranian Convention for the protection of vertebrate animals used for experimental and other scientific purposes, and the protocol was approved by the ethics committee of Guilan University of Medical Sciences, Rasht, Iran (IRB number; 88038).
Body weight and food intake
Body weight, food intake and visceral fat were measured using a scale (Sartorius, Germany) accurate to 0.1 g. All rats were weighed once a week, between 09.00 and 11.30. Also, food intake was monitored weekly. Once a week, the food was weighed before it was given to the animals and again at the same time the next day, after it was removed from the cages. To measure the food intake, an equal amount of food (20 g/day/rat) was given to each rat in each cage in each identical group, and food consumption was measured by subtracting the weight of the remaining uneaten food. The differences between the weights were divided by the number of rats in the cage. After 8 weeks of exercise, rats were anesthetised for the measurement of body length (nose-to-anus).
Vitamin D supplementation
Two month dietary manipulation of vitamin D was carried out using cholecalciferol (Vit D3) added to an isocaloric diet in the following amounts (based on pilot studies intended to mimic the range of human levels): Aerobic training with vitamin D supplementation = 10000 IU/kg (9000 IU by injection and 1000 IU by food intake) per week, vitamin D supplementation = 10000 IU/kg (9000 IU by injection and 1000 IU by food intake) per week, control Vit D = 1000 IU/kg food a week, Aerobic training Vit D = 1000 IU/kg food per week [27].
Protocol for exercise training
Rats in the AT group were trained to run on a motor-driven rodent treadmill (Iranian Model, seven lanes, designed by Sports Sciences Research Center, Tehran, Iran) from the 3rd week of experiment (16 weeks old). During the first week of training, rats began to practice walking at the speed of 6 m/min, 10 min/day, with no inclination of the treadmill. They were then trained to run based on the protocol. Exercise training consisted of running on the treadmill for 3 days/week for 8 weeks. Rats progressed from running for 15 min/day at 15 m/min, 0% slope, up to 40 min/day at 25 m/min, 10% slope for the last 4 weeks [28]. The regular endurance exercise used in this study is equivalent to 70–85% VO2max [29].
Groups and surgery
Two weeks after acclimatization, 16-week-old rats were separated into OVX (n = 32) and sham-operated control (SHAM; n = 8) groups. OVX rats were subdivided into four groups: aerobic training (OVX + AT; n = 8), vitamin D supplementation (OVX + Vit D; n = 8), aerobic training with vitamin D supplementation (OVX + AT + Vit D; n = 8) and control (OVX + C; n = 8) group. Rats were ovariectomized and sham-operated under general anesthesia with an intraperitoneal injection of ketamine (50 mg/ml; Rotex Medica, Germany) and xylazine (20 mg/ml; Alfasan, the Netherlands) at a ratio of 4:1, according to the technique described by Shinoda and colleagues [27,30].
Blood analysis
All trained rats were restrained from training and food was removed from the animals’ cages at least 12 h before sacrifice. After complete anesthesia, the abdominal cavity was rapidly opened and blood samples were drawn from the inferior vena cava. The serum was separated by centrifugation (4000 rpm for 15 min) and stored at −80°C for later biochemical and hormonal measurements.
Assessment of concentrations of plasma lipids
The concentrations of triglyceride [31], total cholesterol [32], HDL-cholesterol [33] and LDL-cholesterol [34] in the plasma were measured using enzymatic analysis kits (Asan pharmaceuticals, Hwasung, Korea). The serum glucose concentration was determined with an enzymatic (GOD-PAP, glucose oxidaseamino antipyrine) colorimetric assay (Pars Azmoun, Tehran, Iran) and the serum insulin concentration was measured with a rat insulin ELISA kit (DRG, USA). HOMA-IR (fasting insulin (mμ/ml)×fasting glucose (mmol/l)/22.5) was used to estimate the insulin resistance. All measurements were perfomed in duplicate [35].
Statistical analysis
All data are presented as mean ± standard error. Before statistical analysis, the normal distribution and homogeneity of the variances were tested. Statistical comparisons between groups were performed by the one-way ANOVA test. Comparisons within groups (after 8 weeks) were performed by repeated measure ANOVA followed by Tukey’s post-hoc test. The SAS software version 9.1 was used for data analysis, with the level of significance set at 5% (P < 0.05).
RESULTS
All of the trained animals successfully completed the 8 weeks of aerobic training. The results for body weight and food intake changes between and within groups are shown in Figs. 1 and 2 respectively. Initial body weights and food intakes in the OVX groups were not significantly different in this study. Compared with the SHAM rats, the OVX rats were found to have higher body weight intakes (P ≤ 0.05). Three weeks after the beginning of the study, there was significant difference in body weight between the groups (Fig. 1). The repeated measures ANOVA test showed a significant reduction in body weight in OVX + AT + Vit D, OVX + AT and OVX + Vit D groups during the experiment, while the body weights of the OVX-C and SHAM groups significantly increased after 8 weeks compared with the initial body weights. Also, food intake significantly increased in the OVX + C and SHAM groups over time, but significantly decreased in the OVX + AT + Vit D group (Fig. 2). Interestingly, the mean body weight and food intake from the third week in the control group was significantly higher than in the other groups (Fig. 1 and 2).
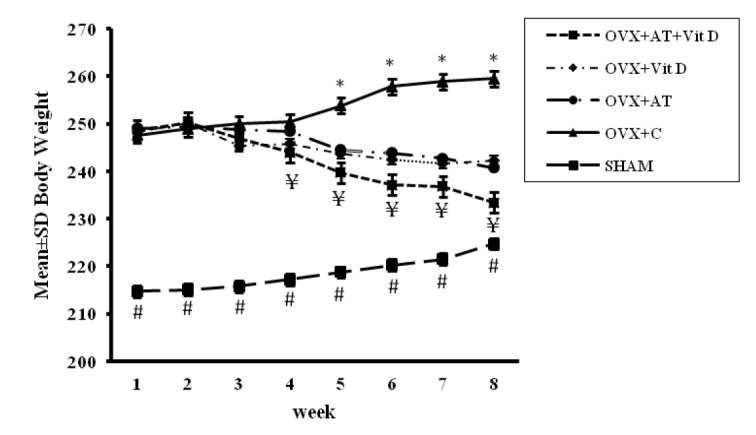
Comparison of mean ± SD body weight between groups. One-way ANOVA test followed by Tukey’s post-hoc test. *Significant differences between the control and other groups (P < 0.001). ¥ Significant differences between the OVX + AT + Vit D and other groups (P < 0.002). # Significant differences between the SHAM and other groups (P < 0.002), the level of significance was set at 5% (P < 0.05); OVX + AT + Vit D, aerobic training with vitamin D supplementation group; OVX + AT, aerobic training; OVX + Vit D, vitamin D supplementation group; OVX + C, control group; SHAM, sham-operated (SHAM).

Comparison of mean ± SD food intake between groups. One-way ANOVA test followed by Tukey’s post-hoc test. *Significant differences between the control and other groups (P < 0.001). # Significant differences between the OVX + AT + Vit D and other groups (P < 0.001), the level of significance was set at 5% (P < 0.05); OVX + AT + Vit D, aerobic training with vitamin D supplementation group; OVX + AT, aerobic training; OVX + Vit D, vitamin D supplementation group; OVX + C, control group; SHAM, sham-operated (SHAM).
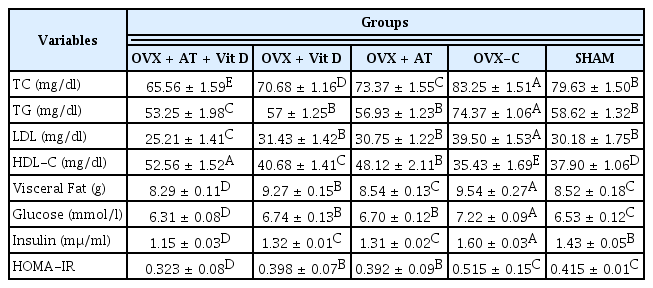
The TC, TG, HDL-C and LDL-C levels are shown in table 1. The TC levels were significantly higher in the C group than in the other groups (11.74%, P = 0.002), while the TC levels were significantly lower in the OVX + AT + Vit D group than in the other groups (11.99%, P = 0.001). The OVX + AT + Vit D group displayed significantly lower TG levels than the other groups (11.30%, P = 0.001). Similarly, the OVX + AT, OVX + Vit D and SHAM groups had significantly lower TG levels than the C group (15.01%, P = 0.001; 1.51%, P = 0.003; 4.34%, P = 0.002). The HDL-C levels were significantly higher in the OVX + AT + Vit D and OVX + AT groups than in the other groups (22.40%, P = 0.001 and 12.06%, P = 0.043) (Table 1). Furthermore, the HDL-C levels were significantly lower in the C group compared with the other groups (17.48%, P = 0.002). The LDL-C levels were significantly lower in the OVX + AT + Vit D group than in the other groups (19.74%, P = 0.001) and were significantly much higher in the C group compared with the other groups (25.74%, P = 0.001). The visceral fat level was significantly lower in the OVX + AT + Vit D, OVX + Vit D, OVX + AT and SHAM groups compared with the control group (13.10%, P = 0.001; 2.83%, P = 0.003; 10.48%, P = 0.004 and 10.69%, P = 0.003, respectively). Glucose, serum insulin and HOMA-IR levels decreased significantly by 5.82%, 15.56% and 20.94%, respectively, in OVX + AT + Vit D compared with the other groups (P = 0.001; Table 1).
DISCUSSION
The purpose of this study was to examine the interaction between aerobic exercise training and vitamin D supplementation and its effect on plasma lipid profiles and insulin resistance in ovariectomized rats. In the study, ovariectomy resulted in a significant increase in body weight and also an increase in visceral fat, confirming previous findings [36,37], This indicates that the lack of estrogen following ovariectomy influences body weight, partially by increasing visceral fat [1,3]. Estrogen plays a crucial role in distributing visceral fat, probably by changing lipoprotein lipase activity [1,3,38]. Our study revealed that exercise training decreases body weight, which has also been reported in many studies [39,40]. On the other hand, there is evidence showing lack of weight reduction after exercise [41,42], especially in menopausal women [43]. It has been reported that the concentration of serum vitamin D has an effect on muscular activity, the number and size of muscular fibers and also the fat free mass [4]. The present study showed that treatment with OVX-AT & Vit D for 8 weeks leads to more weight loss (21%) compared with exercise alone (7%). Additionally, mean food intake from week three in the control group was significantly higher than other groups. Bunout et al. reported that in a study comparing exercise training and vitamin D intake (400 IU/day) over 9 months in elderly women, the fat free mass and the muscle strength in the exercise training group significantly increased, but there were no significant changes in fat free mass and muscle strength in the vitamin D intake group [44]. It has been reported that unlike vitamin D intake, regular aerobic training effects positive changes in body weight [40]. Exercise training targeting patients with diabetes improve their health and has positive effects on body weight [39]. On the other hand, there have also been research results indicating that significant changes in body weight were not apparent despite participation in exercise training. According to body weight and food intake results obtained in this study, the most significant reduction was in the group with aerobic training combined with vitamin D. The mechanism by which an increase in the concentration of vitamin D in the body affects body composition is difficult to explain. However, some studies reported no effect from vitamin D on body weight change and energy expenditure and little improvement in cardiovascular risks with cholecalciferol [45,46]. Interestingly, Vitamin D is fat - soluble and is readily stored in adipose tissue; it is sequestered after absorption and stored in tissues such as fat and muscle [47]. This fate of vitamin D has been demonstrated by injecting radio-labeled vitamin D3 into individuals and monitoring the highest levels of biological activity and radioactivity in the fat tissue [48]. 1,25-Dihydroxyvitamin D, inversely, modulates adipokine expression, inhibits anti-inflammatory cytokine expression, reduces monocyte recruitment by human pre-adipocytes, and restores glucose uptake in adipocytes [49]. 1,25-Dihydroxyvitamin D has a role in human adipose tissue because it is the active form of the vitamin D3 metabolite. Vitamin D receptors (VDR) present in adipocytes allow the suppression of Parathyroid hormone (PTH) levels. The PTH excess observed in menopausal women with both primary and secondary hyperparathyroidism may promote weight gain by impeding catecholamine-induced lipolysis [50]. A previous study showed that Vit D has a positive effect on muscle mass, especially on fat free mass, and also on muscle activity [4]. We assumed that an increase in muscle mass could elevate metabolism and decrease fat mass [51]. Regarding the One-way ANOVA test, we showed that the TG, TC, and LDL-C levels were significantly decreased with increased aerobic training and vitamin D intake. Also, the HDL-C levels were significantly higher in the OVX + AT + Vit D and OVX + AT groups compared with the other groups. Several studies have revealed the importance of nutritional education aimed at preventing Cardiovascular disease (CVD) in postmenopausal women [52,53]. The present study confirmed this with the observation of a significant decline in body weight, food intake, TG, TC, and LDL-C levels as well as increases in the HDL-C level by aerobic training and Vit D. Interestingly, weight loss parallels the reduction of cardiovascular risk factors (TG, TC, LDL-C) and HDL-C levels were improved in the exercise with vitamin D supplemented group compared with the exercise only group. Few studies have investigated the relationship between serum levels of vitamin D and lipid profiles. Major et al. reported that in obese middle-aged women taking 200 IU of vitamin D per day for 15 weeks, TC, LDL-C, and HDL-C concentrations were significantly reduced [54]. Rejnmark and colleagues performed a study among 82 healthy postmenopausal women who had been treated with either 40 mg/day Simvastatin or a placebo for 1 year, in which vitamin D, TG, and LDL levels were measured at baseline and after 26 weeks of treatment [55]. In this study, Simvastatin showed no effect on vitamin D status, but reduced the serum levels of TG and LDL. These results suggest that the serum concentration of TG is inversely associated with the serum level of 25(OH) D [55]. In contrast, Kota and colleagues showed no relationship between serum levels of 25(OH) D and TG or HDL cholesterol in healthy subjects [56]. It was suggested that vitamin D has both direct and indirect effects on modifying the lipid profile and that the effect of vitamin D on decreasing serum levels of TG, TC and LDL may occur through regulatory action that increases the activity of lipoprotein lipase in adiposity [57]. In our study, exercise led to improvements in lipids profile as well. In agreement with our results, Greene et al. also reported that obese middle-aged men and women participating in endurance exercise at 70% of VO2max, 3 times per week for 12 weeks had significant improvements in lipid profiles [58]. Sung & Bae reported a similar finding in diabetic elderly men participating in walking exercise at 65–75% of HRmax for 50 minutes a day, 3 times a week for 24 weeks [59]. Taken together, aerobic training is considered effective for inducing positive changes in blood lipid. Several mechanisms are suggested to explain the effect of vitamin D on lipids, including its reducing role in fatty acid absorption via the formation of insoluble calcium-fatty complexes in the gut. By decreased absorption of fat, particularly saturated fatty acids, it is expected that serum levels of total and LDL cholesterol will be reduced [60]. In addition, vitamin D can increase the conversion of cholesterol to bile acids due to its ability to bind with bile acids [61]. However, the effect of enteric vitamin D and calcium on lipid absorption is very limited and it does not have a significant effect on lipid profiles [62]. On the other hand, in vitro studies have demonstrated that PTH reduces lipolysis (through increasing the level of cytosolic calcium) [63] and increases the expression of fatty acid synthesis [64].
Based on existing studies, it is known that the accumulation of visceral fat in postmenopausal women is the main cause of insulin resistance and metabolic syndrome [65,66]. It has been reported that the accumulation of visceral fat increases the secretion of adipocytokine and has a negative effect on hypertension, diabetes and cardiovascular diseases [67]. Previous studies reported that vitamin D deficiency often appeared in cases of obesity and the concentration of 25(OH)D shows a negative correlation with visceral fat [68]. In our study, visceral fat showed more reduction in OVX + AT + D compared with other groups. The reduction of visceral fat following chronic exercise has been reported in literature [37]. Interestingly, the reduction of visceral fat in the group receiving co-treatment of exercise and vit D was much higher than with exercise alone. In this regard, Bacchi et al. reported that for middle-aged men and women with diabetes conducting aerobic exercise using a tread mill or cycle at 60–65% of HRR, 60 minutes per day, 3 times per week for 16 weeks, the abdominal visceral fat area and subcutaneous fat area were significantly reduced [69]. While inconsistent, Wamberg et al. reported that women taking vitamin D (7,000 IU per day over 26 weeks) showed no significant changes in subcutaneous fat mass and visceral fat mass [70]. Generally, the research results show that aerobic exercise training and adequate intake of vitamin D positively affect the body weight, visceral fat and plasma lipid profiles during menopause and also reduce metabolic syndrome risk factors [70,71].
In addition, the results of our study showed a significant improvement in insulin resistance in the OVX + D + AT group (Fig 2, B and, D). Talaei et al. reported that people who have vit D insufficiency (< 30 ng·ml−1) and people with deficiency (< 20 ng·ml−1) < incomplete sentence> [72]. Also, VonHurst et al. reported that as a result of taking 4,000 IU of vitamin D every day for 26 weeks, the insulin and blood glucose levels of patients with diabetes were significantly reduced. These results suggest that vitamin D intake increases the sensitivity of insulin [73]. Although our previous studies [37,74] and others showed adequate effectiveness by regular physical activity in improving insulin resistance, OVX + AT + D showed a better response [43,75]. There are some mechanisms for the effects of vitamin D: the presence of vitamin D receptors on pancreatic β cells [76], Vitamin D-activating 1αhydroxylase is expressed in pancreatic β cells [77], the vitamin D response element in the insulin gene due to the presence of vitamin D receptors in skeletal muscle [78] and the fact that 1,25(OH)D increases the transcription of insulin receptor genes [79] and also suppresses the renin gene, reducing hyperglycemic-induced increases in renin levels in pancreatic β cells. Blockade of renin-angiotensin activity has been proposed as a novel target for the treatment of diabetes [80]. The protective effects of vitamin D on obesity may be due to well known effects of vitamin D such as its anti-inflammatory properties, its effects on calcium and phosphorus metabolism and its regulation of the insulin receptor gene [79]. It seems that vitamin D increases the calcium content of cells, in turn leading to the increased transport of glucose into the muscle [81]. Vitamin D also regulates nuclear PPAR (Peroxisome proliferative activated receptor), which has an important role in insulin sensitivity [82]. It would be useful though to undertake further studies to discover more about the mechanism and the effect of vitamin D on both alpha and islet beta-cell functions and also on the mechanisms that determine insulin resistance. Aerobic training and vitamin D supplementation contribute to increases in the metabolic rate by improving cardiovascular capacity and suppressing pro-inflammatory cytokine production, in addition to increasing the release of nitric oxide and the consumption of free fatty acids, thus enhancing insulin sensitivity [83]. In general, inadequate vitamin D may promote greater adiposity through other metabolic effects, such as the regulation of PTH and the modulation of adipogenesis. Moderate to severe vitamin D deficiency leads to increased PTH, which may promote an increase in free intracellular calcium in adipocytes and thereby enhance lipogenesis [84,85]. The question that needs to be answered is whether or not vitamin D deficiency prevents the beneficial effects of exercise especially after menopause. If, yes, by what mechanisms could this interaction be explained? Recently, numerous interventional and epidemiological studies have shown that calcium and vitamin D consumption effectively reduce abdominal fat and metabolic syndrome risk [38,86]. It has been reported that vitamin D plays an essential role in the homeostasis control of calcium. Vitamin D was closely connected to the metabolic mechanism of calcium, and increased blood calcium concentration consequently affects blood lipid concentration and insulin resistance. One limitation of the present study was the lack of a group of ovariectomized animals treated with hormone replacement therapy to confirm the positive effects of AT and Vit D on plasma lipid profiles and insulin resistance. Further studies should be conducted to more accurately elucidate and characterize the metabolic energy imposed by this aerobic training protocol and vitamin D supplementation in rats and humans.
CONCLUSIONS
In conclusion, aerobic training and vitamin D supplementation successfully attenuate some metabolic syndrome profiles and might prevent cardiovascular risk factors caused by ovariectomy. From the clinical point of view, this study suggests that vit D supplementation is a very useful strategy to prevent obesity and menopause-associated metabolic disturbances.