INTRODUCTION
It is well known that high eccentric muscle contractions induce muscle damage [1]. Morphological changes including disruptions of sarcomeres, cytoskeletal elements, and sarcolemma are accompanied by muscle damage. Loss of muscle function such as reduced force production, an indirect marker of muscle damage, is followed by delayed-onset muscle soreness (DOMS) and leakage of muscle proteins including myoglobin (Mb) and creatine kinase (CK), into the bloodstream [2,3]. Among these markers of muscle damage, CK activity is one of the markers that show large variability between subjects. This is known as ‘inter-subject variability’ [4,5].
Although the resting value of CK ranges from 45 to 267 U/L [6], it increases over 10,000 U/L following exercise-induced muscle damage [7]. Previous studies reported that CK activity varies after eccentric muscle contractions, ranging from 6,740~24,200 U/L [5], 500~34,500 U/L [8] and 73~82,011 U/L [4]. Many researchers have suggested that the main contributors to inter-subject variability include gender [2,9], age [10], ethnicity [11], training status [12], mode of exercise [13], and genetic factors [14,15]. Body composition has also been a potent contributor to inter-subject variability of CK activity. Paschalis et al. [16] reported that overweight individuals showed higher CK activity following eccentric exercise than those with normal body weight based on the body mass index (BMI). Heled et al. [17] also reported that individuals with higher CK activity after exercise have higher % body fat compared to those with less CK activity. Although several factors such as muscle mass and fat mass have been suggested to explain the relationship between body composition and CK variability, other factors need to be clarified.
Since CK activity in the blood is believed to reflect muscle membrane disruption [18], maximal isometric strength and muscle soreness following eccentric muscle contractions might be correlated. However, several studies have reported that there is no clear correlation between increased CK activity and decreased maximal isometric strength or muscle soreness [19,20]. Despite the results from these studies, small sample sizes were used to evaluate the relationship. Therefore, the purpose of the study was to investigate the relationship between CK activity and body composition as well as muscle damage markers including maximal isometric strength and muscle soreness in a larger population.
METHODS
Subjects
A total of 119 healthy collegiate male subjects participated in the study. They did not attend any resistance training within the previous 6 months, had no neuromuscular diseases and took no medications or dietary supplements. Subjects that did not meet these requirements were excluded from the study. Each subject was familiarized with all the procedures of the study prior to signing the informed consent form. The study was approved by the Institutional Review Board at Kookmin University, Seoul, Korea.
Each subject was assigned to one of the following groups after eccentric muscle contractions based on their peak CK activity [18]: high responder (> 2,000 U/L), medium responder (500-2,000 U/L), or low responder (< 500 U/L) groups. High responders contained 66 subjects, medium responders had 26, and low responders had 27. We only analyzed high and low responder groups in the study. Thus, 93 subjects were included in the study. The characteristics of each group are shown in Table 1.
Body composition
Anthropometric measures including height, body weight, % body fat, muscle mass, and body mass index (BMI) were obtained using an anthropometer (DS-102, Dongsan, Korea) and bioelectrical impedance (InBody 520, Biospace, Korea). All the measurements were taken on the first day of the study. Each subject was asked to abstain from severe exercise or using a sauna and to fast overnight to obtain consistent measurements during the study.
Eccentric exercise
In this study, the elbow flexor model was used for eccentric muscle contraction [21-23]. Each subject placed his non-dominant arm on the pad attached to a modified preacher curl machine with the elbow joint at 90 degrees. When the subjects pulled the pad toward their trunk, the investigator pulled down on the lever attached to the pad in a direction opposite the subjects. In this way, eccentric muscle contractions were induced in the subjects’ elbow flexor. Each contraction lasted for 3 seconds followed by 12 seconds of rest. Eccentric muscle contractions were performed with 2 sets of 25 contractions.
Maximal isometric strength
Maximal isometric strength (MIS) was measured using a strain gauge (PKS-1250, Poong Kwang, Korea) attached to a modified preacher curl machine. Each subject’s non-dominant arm was placed on the pad of the preacher curl machine with the elbow joint at 90 degrees. Then, when subjects pulled the pad maximally toward to their trunk, MIS was measured pre and post, and at 24, 48, 72 and 96 hours after exercise. Three trials were attempted and averaged to calculate % MIS. The pre-value for MIS was set at 100%. The post-value of MIS was used to identify whether each subject performed maximally during eccentric muscle contractions. When post MIS was less than 30% of baseline (pre MIS), subjects were excluded from the study.
Muscle soreness
Muscle soreness (SOR) was measured using a visual analog scale (VAS). On the VAS, a 100 mm horizontal line was drawn, with 0 as no soreness and 100 as very sore. Each subject drew a vertical line on the VAS indicating how they felt around the elbow flexor area. SOR was recorded pre and at 24, 48, 72, and 96 hours after exercise.
CK activity
5 mL of blood samples were taken from the antecubital vein to analyze CK activity pre and at 24, 48, 72 and 96 hours after exercise. Each sample was allowed to sit for 20 minutes at room temperature and then centrifuged (2500~3000 rpm for 15 minutes) to separate the serum. The serum samples were stored in a -80 degree freezer prior to analyzing the CK activity using an automated blood analyzer (VITROS DT60II, Kodak, USA).
Statistical analyses
All variables were presented as mean and standard deviation. To evaluate the effect of CK variability on muscle damage markers, 2 × 5 factorial repeated measures ANOVA was used and Tukey’s test was used as a post-hoc test when there was significant group-by-time interaction between variables. To evaluate the effect of CK variability on body composition, one-way ANOVA was used. Additionally, Pearson correlation was used to detect the relationship between peak CK activity and muscle damage markers with respect to the time course and body composition. Spearman correlation was also used to detect the relationship between the peak CK value and peak SOR value as well as the lowest % MIS value. The level of significance was set at p<0.05.
RESULTS
Effects of CK variability on muscle damage markers
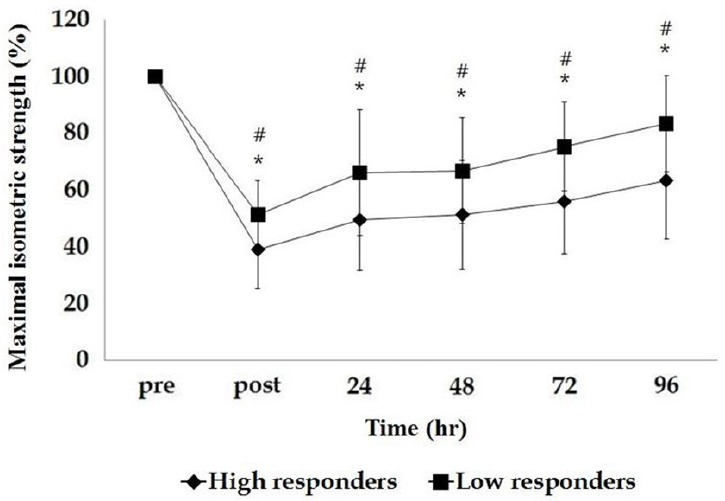
There was a significant group-by-time interaction between CK variability and MIS (p<0.001) by repeated measure ANOVA. There was a significant group effect (p<0.001) and time effect (p<0.001). MIS was significantly different in the low responder group (47% decrease of baseline) immediately after exercise compared to the high responder group (62% decrease of baseline) indicating that the low responder group showed less initial muscle damage. In addition, the low responder group demonstrated faster MIS recovery after exercise by 96 hours compared to the high responder group, indicating that the lower responder group might have less muscle damage as shown in Fig. 1.
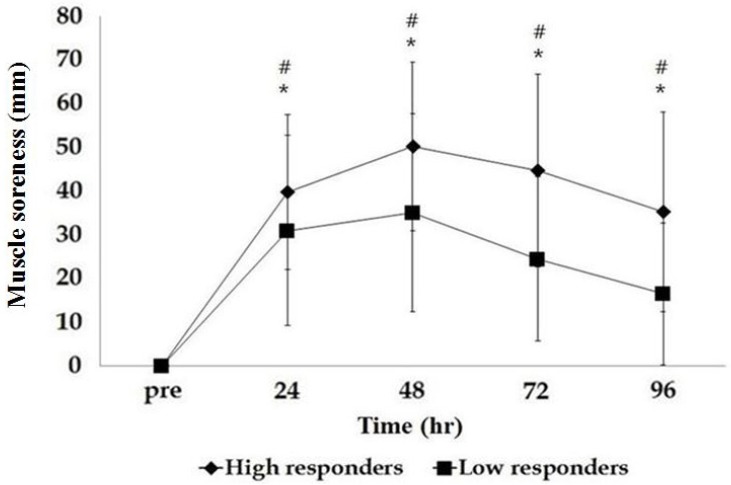
There was a significant group-by-time interaction between CK variability and SOR (p<0.01) analyzed by repeated measures ANOVA. There was also a significant group effect (p<0.001) and time effect (p<0.001). Both groups demonstrated significant increases in SOR after exercise but the low responder group had less SOR compared to the high responder group as shown in Fig. 2.
Effect of peak CK activity on body composition
According to one-way ANOVA analysis, there were significant differences in % body fat (p=0.006), muscle mass (p=0.047), and BMI (p=0.001) among the three groups. The medium responder group had significantly higher % body fat, muscle mass, and BMI compared to the low and high responder groups. When the medium responder group was eliminated, there was a significant difference in % body fat, with the high responder group showing higher % body fat compared to the low responder group (p=0.014). However, there were no significant differences in muscle mass (p=0.25) and BMI (p=0.135) between the low and high responder groups.
Correlation between peak CK activity and muscle damage markers and body composition
Correlation data are presented in Tables 2 and 3. There was a significant correlation between peak CK and MIS, analyzed by Pearson correlation as shown in Table 2. However, there was no significant correlation in SOR using Pearson correlation, although Spearman correlation indicated a significant correlation. Additionally, there was no significant correlation between peak CK and body composition variables including % body fat, muscle mass, and BMI as shown in Table 3.
DISCUSSION
This study was performed to elucidate the relationship between CK variability and muscle damage parameters including maximum isometric strength and muscle soreness as well as body composition following eccentric muscle contractions. It is well documented that eccentric muscle contractions induce morphological changes including Z-disk streaming and sarcolemma disruption, leading to increased membrane permeability and subsequent leakage of CK into the circulation [24]. The measurement of CK activity, therefore, has been used as an indicator of membrane integrity after muscle damage. The factors that affect membrane integrity include estrogen, dystrophin, and genotypes. In general, females show lower CK activities than males due to the protective effect of estrogen on cell membranes [25,26]. Stupka et al. [26] reported lower CK activity in females 48 hours after eccentric exercises with leg presses. Since dystrophin is also known to be related to membrane integrity and individuals with dystrophin deficient conditions such as Duchene’s or Becker’s muscular dystrophy showed higher CK activity up to 25~200 fold [27]. Yamin et al. [15] reported lower CK activity in the DD allele compared to the II allele for ACE polymorphisms following eccentric muscle contractions. Funghetto et al. [28] reported higher CK activity in the GG allele for interleukin-6 -174G/C polymorphism following eccentric exercise.
In early studies, CK activity was closely related to the magnitude of muscle damage [8], while later studies demonstrated that CK activity was not correlated with the degree of muscle damage [29]. Later studies biopsied the vastus lateralis muscle after high-force eccentric exercise in young and old males and then observed morphological changes using an electron microscope [29]. The authors concluded that older individuals had greater morphological disruption compared to young individuals but there was no significant difference in CK activity between the two groups. Several studies have also reported no significant relationship between CK activity and maximum isometric strength. Margaritis et al. [30] demonstrated that maximum isometric strength returned to baseline at 4 days after competition but CK activity continued to increase when muscle damage markers were analyzed among triathlon athletes after competition.
These results have raised the question of whether CK activity is a sensitive marker of muscle damage. However, several recent studies showed a significant relationship between peak CK activity and maximum isometric strength. Totsuka et al. [31] reported that there is a negative relationship between peak CK activity and muscle strength in the leg extensors. Additionally, Nosaka et al. [20] reported that maximum isometric strength immediately after eccentric exercise is negatively correlated with peak CK activity. Therefore, most studies to date have shown controversial results but the sample sizes were small.
In this study, we used a relatively larger sample size compared to previous studies and could confirm peak CK activity and other muscle damage markers as well as maximum isometric strength. We found a significant difference in maximum isometric strength based on CK variability, where high responders showed a greater decline in maximum isometric strength immediately after exercise and a slower recovery compared to low responders. The results also indicated a significant negative relationship between peak CK activity and maximum isometric strength following eccentric exercise. This may suggest that peak CK activity is closely related to the magnitude of muscle damage. Nosaka and Clarkson [32] reported that the T2 relaxation time for magnetic resonance imaging is higher in individuals with high CK activity after eccentric exercise and Evans et al. [33] also reported that peak T2 relaxation time coincided with peak CK activity. Additionally, Nurenberg et al. [34] reported that T2 relaxation time is negatively correlated with normal muscular structure and function, suggesting that both CK activity and T2 relaxation time could be markers for muscle function.
In the current study, there was a significant difference in muscle soreness based on CK variability, with high responders showing higher muscle soreness compared to low responders. Although the Pearson correlation did not show a significant relationship between peak CK activity and muscle soreness in the time course after eccentric exercise, Spearman correlation showed a significant relationship between the two variables. Previous studies have reported no relationship between CK activity and muscle soreness following eccentric exercise [19,35]. A recent study however, reported that there was a significant relationship between myoglobin levels and the number of neutrophils as well as between myoglobin levels and muscle soreness [36]. Myoglobin is also an indirect blood marker of muscle and cell membrane damage while neutrophil is known to induce an inflammatory response, leading to muscle soreness [37]. Although the mechanism is not well elucidated, it may be that the inflammatory response following muscle damage increases CK activity as well as muscle soreness.
Finally, when body composition was compared with CK variability, high responders had higher % body fat compared to low responders. Several studies reported that body fat is a potent factor in muscle damage [16,17]. Heled et al. [17] reported higher CK activity in individuals with high body fat after eccentric exercise. Paschalis et al. [16] also reported that overweight females with an average of 31% body fat and 29.5 body mass index (BMI) showed higher CK activity after eccentric exercise compared to individuals with normal % body fat and BMI.
Although the relationship between body fat and muscle damage is not fully clarified, the mechanisms can be inferred from several obesity studies. Kriketos et al. [38] reported that obese individuals with high % body fat had more type II muscle fibers in biopsied muscle compared to non-obese individuals. It is well known that type II fibers are more prone to damage than type I fibers [39,40]. In addition, obese individuals generally had reduced physical activity, leading to less exposure to eccentric muscle contractions. Therefore, obese individuals showed more muscle damage due to less adaptation to eccentric contractions [16]. In an animal study, obese mice showed greater myofiber membrane disruption after eccentric exercise compared to lean mice [41]. However, since the subjects in this study did not show excessive body fat, the aforementioned factor provides limited explanation. The effect of body fat on muscle damage requires further investigation.