Effect of different training mode on Interleukin-6 (IL-6) and C-reactive protein (CRP) in type 2 diabetes mellitus (T2DM) patients
Article information
Abstract
[Purpose]
This study was carried out to investigate the effects of different training modes on IL-6 and CRP in patients with type 2 diabetes mellitus (T2DM).
[Methods]
The subjects consisted of 16 middle-aged women with type 2 diabetes mellitus (T2DM), all of whom had no other complications. The 16 subjects were randomly assigned to two experimental groups: the circuit training group (CTG, n = 8) and aerobic training group (ATG, n = 8). Based on measured THR (target heart rate) for maximum oxygen consumption rate, the circuit training group (CTG) exercised at 60% intensity, 60 min/day, 5 sets, 3 days/week for 12 weeks. Based on measured THR (target heart rate) for maximum oxygen consumption rate, the aerobic training group (ATG) exercised at 60% intensity (which was increased gradually in weeks 4, 7, and 10) 60 min/day, 3 days/week for 12 weeks.
[Results]
The results are as follows. Significant decreases in the post training values of weight, % Fat, BMI, IL-6 and CRP (p < .05) were observed in the CTG compared to pre-training. However, there were no differences in the physical characteristic and blood inflammatory factors between the groups (ATG & CTG).
[Conclusion]
In conclusion, the results of this study suggest that circuit training (CT) may be considered as an effective training mode for helping to decrease the blood inflammatory factors (IL-6 and CRP) in patients with type 2 diabetes mellitus (T2DM).
INTRODUCTION
Weight loss and physical activity are effective for the prevention of metabolic syndrome (MS) and type 2 diabetic mellitus (T2DM) [1]. In addition, chronic low levels of inflammatory factors are associated with obesity and sedentary lifestyle, and are independent risk factors for T2DM and cardiovascular disease (CVD) [2].
Exercise training is known to be inversely related to increased levels of pro-inflammatory markers and promotion of insulin resistance. The steady movement of muscles also induces anti-inflammatory effects [3].
Atherosclerosis and coronary heart disease are associated with elevated levels of interleukin 6 (IL-6), C-reactive protein (CRP) and tumor necrosis factor (TNF-α). Elevation of the inflammatory factors IL-6 and CRP in T2DM with metabolic dysfunction [4-6], MS or obesity play a key role in insulin resistance [7]. Chronic low-level inflammatory factors ncrease the levels of cytokines and CRP. Conversely, physical activity increases the levels of anti-inflammatory cytokine [3]. Recently, this cytokine has been called myokine because it is released from skeletal muscles, as well as endocrine tissues [3,8]. The levels of cytokines tend to be elevated more at higher intensity in acute exercise [9]; however, it was proven that the levels are reduced by regular aerobic training [2,10].
Of the many training modes, aerobic training has generally been recommended for patients with T2DM. However, the American Diabetes Association [11] and American College of Sports Medicine [12] recently suggested that a combination of aerobic and resistance training is more effective for managing diabetes. Thus, the effects of combined resistance and aerobic training have also been studied [13,14], and combined training was proven to be effective for reducing plasma cytokine levels, similar to the effects of aerobic training. When only resistance training was conducted 3 days per week for 12 weeks [15] or 3 days per week for 40 weeks [16], the plasma cytokine levels (IL-6, CRP) were not significantly reduced. Another mode of training, circuit training, consists of an alternating series of aerobic and resistance exercises, and is proven to have effects for improvement of physical fitness and promotion of health [17]. Moreover, there are differences between circuit training and combined training. While aerobic training proceeds with resistance training in the combined training, circuit training involves both aerobic and resistance exercises, and prevents boredom by mixing the two up [13]. Accordingly, circuit training is able to compensate for the disadvantages of resistance or aerobic training. In this regard, it is thought to be difficult for diabetes patients to perform combined training because different two types of exercises should be done during the training. However, resistance training can contribute to improving the impaired glucose tolerance (IGT) and insulin sensitivity in T2DM patients, and low-intensity resistance exercise is recommended in circuit training [18]. Through this training, muscle strength and cardiovascular endurance can be improved at the same time. Therefore, it was determined that circuit training would be appropriate for patients with T2DM, and it was selected for examination in the present study [19]. The advantages of circuit training are also thought to influence inflammatory factors. However, few studies have been done on the effects of circuit and aerobic training on inflammatory factors, compared to single or combined training.
The present study aimed to suggest an effective exercise mode for T2DM patients by identifying the effects on inflammatory factors according to two different training modes: 12 weeks of moderate-intensity circuit and aerobic training.
METHODS
Subjects
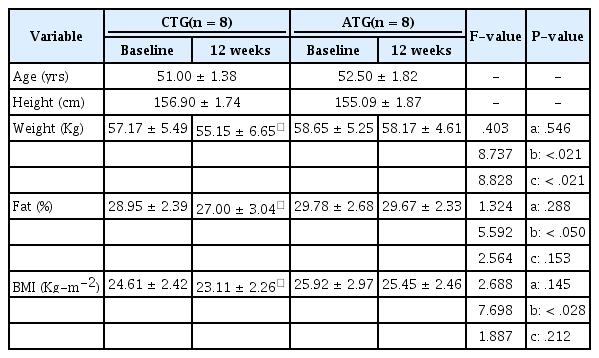
Of the female T2DM patients who were admitted to D University Hospital, 16 patients who understood the purpose of the study and agreed to participate were randomly selected. They were asked to choose their preferred training program between the two different training programs offered (circuit and the aerobic training). The inclusion criteria for this study were as follows: (1) patients without severe disease or other serious complications of diabetes, (2) patients with HbA1c ranging from 7 to 10%, (3) patients without renal dysfunction, and (4) patients with fasting blood glucose levels of 200 mg/dl or less. The patients were divided into two groups: the circuit training group (CTG, n = 8) and the aerobic training group (ATG, n = 8). The physical characteristics of the patients are described in Table 1.
Anthropometric assessment and body fat measurement
For height, weight, BMI and body fat assessment, measurements were carried out at rest using a body composition analyzer (Venus 5.5, Jawon Medical), before and after the 12-week training program. Body mass index (BMI) is defined as the weight in kilograms divided by the square of the height in meters.
Maximal-graded treadmill exercise test (maximal-GTX) and 12 weeks of circuit and aerobic training program
The maximal-graded treadmill exercise test (maximal-GTX) is one of the treadmill (Model Q65, Quinton Co, U.S.A) exercise tests designed by Bruce (1973). Herein, the subjects began with 3 minutes of treadmill exercise at 1.7 mph (mile per hour) and at a) gradient of 10%. At three minute intervals, the incline of the treadmill was increased by 2%, and the speed was also increased to 2.5 mph, 3.4 mph, 4.2mph and then 5.0 mph.
Peake et al. (2005) reported that the intensity of exercise contributing to the effective reduction of plasma cytokine was 60% VO2max [20]. In this study, the maximal oxygen consumption rate (VO2max) was measured in all patients during the maximal-GTX with the Bruce protocol. Based on the measured maximal oxygen consumption rate, the target heart rate (THR) corresponding to 60% VO2max was calculated for each participant [21].
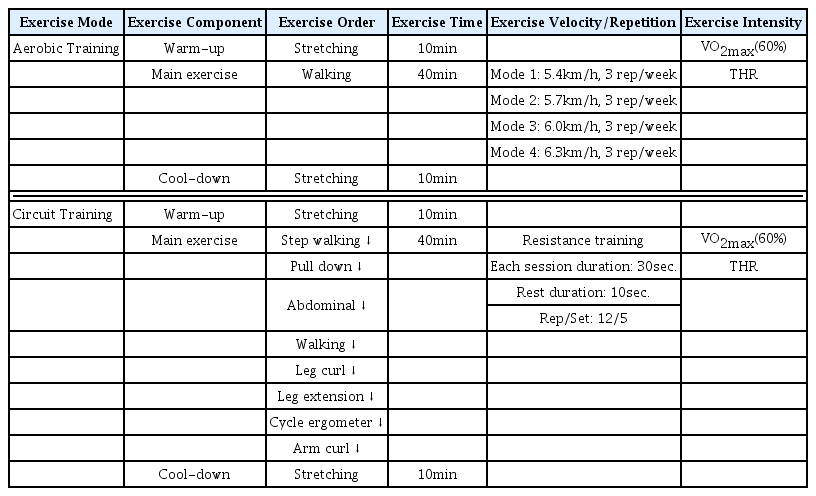
The circuit training group (CTG) wore a Polar heart rate monitor and exercised at a THR corresponding to 60% VO2max for 60 minutes per day, 3 days per week, for 12 weeks. The circuit training program began with a 10-minute warm-up, and the main workout consisted of 5 sets at 12 RM (Repetition Maximum), carried out in the order of Step walking, Pull down, Abdominal, Walking, Leg curl, Leg extension, Cycle ergometer and Arm curl. Each exercise was performed for 30 seconds and interspersed with 10-second rest periods. A total of 5 sets with 2 minutes of rest between the sets were carried out for 40 minutes. Finally, cool-down exercise (stretching) was performed for 10 minutes. Completion of the circuit training program took a total of 60 minutes, and hypoglycemia did not occur during the exercise.
The aerobic training group (ATG) also wore a Polar heart rate monitor and exercised at a THR corresponding to 60% VO2max for 60 minutes per day, 3 days per week, for 12 weeks. Warm-up and cool-down exercises were performed for 20 minutes, and a walking exercise, the main workout, was carried out on an eight-lane (450 m) track as follows: For weeks 1-3, the walking was performed at a speed of 5.4 km/h for 40 minutes (walking session 1). For weeks 4-6, the walking was done at a speed of 5.7 km/h for 40 minutes (walking session 2). For weeks 7-9 weeks, the participants walked at a speed of 6.0 km/h for 40 minutes (walking session 3). For weeks 10-12 weeks, the exercise was carried out at a speed of 6.3 km/h for 40 minutes (walking session 4). In summary, the walking speed was increased by 0.3 km/h in three week intervals during the training program. In addition, GXT was conducted every three weeks and the THR corresponding to 60% VO2max was re-set according to the results and applied to the program. It took a total of 60 minutes to complete the walking exercise program, including the warm-up and cool-down exercises. Both training groups (CTG, ATG) performed GTX every 3 weeks, and the THR for both groups was modified based on the results <Table 2>.
Measurement of blood inflammatory factors
Blood samples were obtained from main veins at rest, in a fasting state 10 hours before the GTX test. Sampled blood and plasma were stored frozen at -70℃ until IL-6 and CRP analysis.
CRP was analyzed by a turbidity method based on latex agglutination with an automatic analyzer (Hitachi 7020 analyzer, Japan) and CRP-Latex(II)X2 reagent.
IL-6 was measured by competitive enzyme-linked immunosorbent assay with an ELISA kit (R & D System Minneapolis, MN), according to the manufacturer's instructions. Briefly, anti-IL-6 monoclonal Ab was employed in microwells for the measurement of IL-6 in the samples. The color was measured at the absorbance of 450nm after reacting with biotin-conjugated IL-6 and treatment with streptavidin-HRP.
Statistical analysis
The results of the physical characteristics and variables for inflammatory factors in each group were presented as means and standard deviation using SPSS/PC Windows 18.0. In addition, the differences between groups and between the results before and after the 12-week exercise programs were analyzed using two way-ANOVA by repeated measure. If the main effect or the interaction was significant, paired t-test was used for the analysis of differences between time points in the same group, and independent t-test was used for the analysis of differences in time points between two groups. All statistical significance levels (α) were set at .05.
RESULTS
Physical characteristics
No significant differences in body weight (kg) were found between the two groups. However, a main effect between time points was observed, (F = 8.737, p < .05) as well as a significant interaction between group × time point (F = 8.828, p < .05). Analysis of the interaction revealed a significant reduction in weight of the CTG after the 12-week training, compared to before (p < .05). Weight also tended to be reduced after the 12-week training in the ATG; however, the difference was not significant.
While body fat percentage (% Fat) did not show significant differences between the two groups, a main effect was shown between the time points for the CTG (F = 5.5592, p <.05). However, the interaction of group × time point was not significant.
There were no significant differences in BMI between the two groups; however, a main effect was presented between the time points for the CTG (F = 7.698, p < .05). The interaction of group × time point was not significant <Table 1>.
Changes in IL-6 and CRP
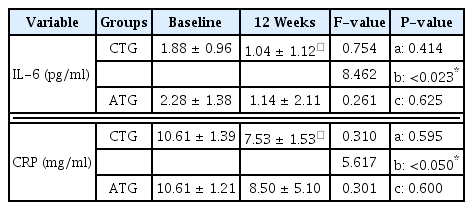
Changes in blood inflammatory factors are described in <Table 3>. There were no significant differences in IL-6 between the two groups; however, there was the significant main effect between time points for the CTG (F = 8.462, p < .05). There were no significant differences in the interaction of group × time point.
There were also no significant differences in CRP between the groups; however, there was the significant main effect between time points for the CTG (F = 5.617, p <.05). There were no significant differences in the interaction of group × time point.
DISCUSSION
IL-6 is classified as an inflammatory cytokine, and elevated levels of plasma IL-6 are commonly observed in MS such as T2DM and CVD. It has been proven that higher-intensity acute exercise leads to an increase of plasma IL-6 in skeleton muscles, which in turn causes an increase of anti-inflammatory cytokines, including IL-1ra, IL-10 and tumor necrosis factor (TNF-α), in the human body [3, 8].
Many studies have been carried out on training programs. Oberbach et al. (2008) reported that plasma IL-6 and CRP levels were significantly decreased with a reduction in BMI by 60-minute endurance training carried out by middle-aged men and women twice weekly for 12 months [10]. Christiansen et al. (2010) also reported that the levels of IL-6 and MCP-1 were significantly decreased after training compared to before in adult men and women as a result of performing 60- or 75-minute endurance training with dieting 3 days per week for 12 weeks [2].
Kohut et al. (2006) compared the different modes of exercises in their study. Patients with MS were divided into the aerobic training group and the resistance/ flexibility training group, training was conducted for 45 minutes per day, 3 days per week, during 10 months. Comparison of the results from the two groups revealed significantly higher decrease in the levels of CRP, IL-6 and IL-18 levels in the aerobic training group than in the resistance/ flexibility training group [16]. Another study on the combination of aerobic and resistance training found significant decrease in the levels of IL-6 and CRP with reduction in body weight and body fat percentage after 8 weeks of combined training [14], which also occurred after 12 weeks of a combination of aerobic and resistance training [13]. However, no significant differences in IL-6 and CRP levels were found when the elderly group conducted resistance training 3 times a week for 12 weeks, for which it was suggested that 12 weeks was too short a period to represent changes at the molecular biological level [15]. In addition, a study on obese subjects comparing 12 weeks of aerobic training and circuit training reported that body weight and body fat percentage were significantly reduced in the circuit training group [22].
In the present study, body fat percentage (% fat) and BMI did not show an interaction in both groups. Significant reductions were observed only within the CTG. However, body weight showed an interaction in both groups, with significant differences in the CTG compared to the ATG. Accordingly, this provides evidence that the circuit training was more effective than the aerobic training. This result also corresponds to that reported by Jung et al. (2008) [22].
Due to the body weight loss, decreases of the IL-6 levels were observed in both groups. Moreover, a significantly decrease (p < .05) occurred in the CTG group, which also showed statistically significant differences in the reduction of body weight and body fat percentage. This was thought to be because the body weight loss induced by the effect of exercise affected the reduction of levels of IL-6, a pro-inflammatory factor [2, 10], and the loss of adipose tissue caused by the reduction of body weight, BMI and body fat percentage affected the gene expression and the consequent production of circulating inflammatory factors [23-25].
While the levels of IL-6 tended to be elevated at higher intensity of acute exercise (Knee-extensor, cycling, running, resistance exercise) [26], they are likely to be reduced by regular and prescribed training.
One of the most important functions of IL-6 recently identified is the role as a myokine, involved in energy metabolism [8]. In this role, as the endocrine system works to maintain glucose homeostasis in the liver, IL-6 contributes to the carbohydrate utilization rate in the muscles during exercise [27]. Plasma IL-6 levels were also reported to decrease through training, eventually caused by decreased ROS and heat shock protein formation [28]. This is a well-known a method of plasma IL-6 gene transcription. However, it is difficult to clearly define the theory mentioned above, because circulating plasma IL-6, not plasma IL-6 released from muscle cells, was measured in this study. In addition, estimation of ROS and heat shock protein was also not carried out herein. Nevertheless, it is true that body weight and body fat were reduced by the training in both groups, and that reduced IL-6 led to a decrease of carbohydrate utilization rate and activation of fat oxidation. In this regard, the theory is considered to be acceptable. Moreover, in terms of the intensity or the mode of training, the optimum training intensity of 60% [20] and the circuit training mode composed of adding resistance factors to aerobic training was believed to have more influence on fat oxidation and weight loss by giving more frequent stimulation to the muscles.
CRP is an indicator of systemic inflammation, and increased CRP during obesity is thought to be caused by IL-6 derived from adipose tissues. This increased CRP can be significantly reduced by applying aerobic training with dieting [29]. In addition, coronary artery disease (CAD) and atherosclerosis were also found to be associated with increased CRP, IL-6 and TNF-α, and these increased values were observed in those suffering from T2DM with metabolism dysfunction, MS or obesity [6]. Physical activity was inversely related to increased inflammatory factors, and the weight loss facilitated by exercise and diet was proven to be associated with decreased plasma CRP, IL-6 and IL-18 levels [30].
In terms of the effects of training, CRP levels were reported to be increased after high-intensity acute exercise [31]; however, the levels were lower after regular and prescribed training, compared to the control group [14]. As a result of applying different training modes, the plasma CRP levels were also reported to be significantly decreased with reduction of BMI 60-minute endurance training carried out twice weekly for 12 months by middle-aged men and women [10]. However, plasma CRP was not significantly decreased by flexibility/resistance training [1] and resistance training alone [32].
In the present study, there were no significant differences in the concentration of CRP between groups; however, there was a main effect between time points, similar to the results of IL-6 levels (F = 5.617, p < .05). The interaction of group × time point was not significant. This means that there were no significant differences in CRP between the two different training modes. However, there was the significant reduction between the start and end of training in the CTG group. As reported in previous studies [10,13,14,16], this reduction also appeared after regular training, and is thought to be due to the decomposition of fat caused by the reduction of weight and body fat. With this result, the mechanism that the secretion of IL-6, an adipocytokine secreted from adipocytes, is increased, which then leads to an increase of lipolytic enzyme and insulin resistance, finally enhancing the production of CRP, an indicator of inflammatory reaction, is thought to be persuasive. The body fat percentages of all subjects in this study ranged from 28% to 29%. Considering their ages, they were regarded as obese with T2DM. Like IL-6, body weight and body fat were significantly reduced in the CTG after the 12 weeks of training (p < .05). In addition, there were significant differences in the CRP levels due to the reduction of body weight and body fat.
Considering that IL-6 promotes the production of CRP [32] and that body weight loss lead to a decrease of CRP, this is the reason for the significant decrease of IL-6 levels in the CTG, whose body weight and body fat were both significantly reduced.
Consequently, it was determined that there were no differences in the physical characteristics (body fat percentage, BMI), except body weight, or in the inflammatory factors (IL-6, CRP) between the two training modes. This means that the circuit training was effective for weight loss; however, differences in the reduction of other inflammatory factors between two training modes were not observed. Therefore, various approaches, for example, a comparison of combined training (aerobic and weight training) and circuit training in T2DM patients, as well as a comparison of different exercise intensities, are required to verify the effects of circuit training in further research.
CONCLUSIONS
This research aimed to suggest a more effective exercise method for T2DM patients who are at risk of blood vessel inflammation by analyzing the differences in body composition and blood inflammatory factors between subjects who carried out 12 weeks of aerobic training (AT) and 12 weeks of circuit training (CT) combined with resistance exercise.
In the physical characteristics and inflammatory factors of the two groups, there were no interactions for body mass index (BMI), body fat percentage (% Fat), IL-6 and CRP. In contrast, there was the significant interaction for body weight between the two groups. Analysis of the interaction revealed that body weight measured after completing the 12 weeks of training was significantly reduced in the CTG, compared to the ATG (p <.05).
In conclusion, the physical characteristics and blood inflammatory factors were decreased by the moderate intensity CT in T2DM patients; however, this training did not provide a significant decrease of the IL-6 and CRP levels, pro-inflammatory factors which may cause the blood inflammation, compared to the AT. Therefore, moderate intensity CT which includes resistance exercise does not have an influence on effectively reducing the physical characteristics and blood inflammatory factors in T2DM patients as compared to the AT mode of exercise.