Effects of exercise with or without light exposure on sleep quality and hormone reponses
Article information
Abstract
[Purpose]
The objectives of the present study were to determine the effect of sun exposure and aerobic exercise on quality of sleep and investigate sleep-related hormonal responses in college-aged males.
[Methods]
In this study, the cross-over design was utilized. The subjects (N = 10) without any physical problems or sleep disorders participated in the experimental performed 4 protocols in only sun exposure (for 30 minutes, EG1) protocol, only aerobic exercise (walking and jogging for 30 minutes, EG2) protocol, aerobic exercise with sun exposure (EG3) protocol, and control (no exercise and no sun exposure, EG4) protocol. Each protocol was 5 times per week with one-week break (wash-out period) between protocols to prevent the effects of the previous protocol. Total test period was should be 7 weeks (one week of protocol and one week of break). Before and after each aerobic exercise session, the subjects completed stretching to warm up for 5 to 10 minutes. Surveys consisting of (bedtime, wake-up time, sleep onset latency, and (Pittsburgh Sleep Quality Index (PSQI) were obtained before the test and after each protocol. After each protocol, the following sleep-related hormonal responses were measured: blood concentrations of melatonin, cortisol, epinephrine, and norepinephrine. One-way ANOVA was used to determine differences between protocols. Statistical significance was set at p < 0.05.
[Results]
Bedtime of EG4 was significantly later than that of the EG1 or EG3. Wake-up time in the EG4 was significantly later than that of the EG1 or the EG3. Sleep onset latency in the EG4 was longer than that of the EG3. The quality of sleep in the EG4 was lower than that of the EG3. Sleep cycle in the EG4 was significantly shorter than that of the EG1. Blood melatonin concentrations of the EG3 was significantly higher than that of the EG4. There were no significant differences in blood concentrations of cortisol, epinephrine, or norepinephrine among protocols, with the order from the lowest to the highest values of EG1 < EG2 < EG3 < EG4.
[Conclusion]
The present data found that EG1 and EG3 showed positive sleep-related hormonal responses, sleep habits, and quality of sleep, indicating that sun exposure or exercise with sun exposure may improve the physical status and quality of life.
INTRODUCTION
Sleep patterns are influenced by biological factors such as age, sex, and circadian rhythms [1]. As we enter the phase of adolescence through childhood, our bedtime gets later, and our amount of sleep gets smaller. After the adolescence, the amount of sleep increases with age and bedtime gets moved forward. Women between 50 and 70 years old have a tendency to have more amount of sleep because they go to bed earlier and get up later than men in the same age range. They frequently wake up from their sleep with longer awake time [2-3].
Sleep plays a direct role in physical and mental recovery, memory, learning, growth, and lifespan. Proper average amount of sleep is known to be 8 hours. In regard to sleep, human body has a circadian rhythm of a 24-hour cycle. Insomnia or hypersomnia can be caused by irregular sleep patterns, anxiety, stress, overwork, excessive exercise, jet lag, and endocrine disorders in the pineal gland [40].
Sleep patterns can be changed by social, environmental, and psychological factors other than biological factors. University students may have irregular sleep-wake cycle due to late bedtime following social or academic requirements [5]. A lifestyle can also affect sleep patterns. The larger the allowance of a female university student is, the later she goes to bed [6]. The quality of sleep gets lowered by smoking, drinking, or caffeine intake [7]. Besides, an individual’s subjective physical conditions are related to sleep patterns [8]. Generally, in terms of biochemistry of the endocrine system, the importance of melatonin secreted from the pineal gland has been emphasized in sleep disorders [9]. Melatonin as a pineal hormone is related to sleep. It has a clear rhythm of secretion. Melatonin is secreted at the highest level in the blood at night. It is rarely released during the day. The melatonin secretion is regulated by the stimuli of neurons in the suprachiasmatic thalamic nucleus that controls mammalian biological cycles [10-11]. Besides, melatonin has been reported to play important roles in controlling nocturnal and diurnal mammalian biological cycles [12]. Human melatonin can induce momentary hypothermia [13-14], hypnosis [15-18], and changes in biorhythms [19-20]. It is closely related to the beginning of sleep as well as the maintenance of sleep [21-22].
Exercise has been suggested to be a method to enrich the quality of sleep [23]. Several studies with healthy volunteers reported that acute and long-term exercises increased slow-wave sleep and total sleeping time while reducing the lack of sleep [24-25]. However, the finding that exercise helps sleep is not accepted world-wide [26]. Several domestic researches dealing with the relationship between sleep and exercise have been conducted on subjects such as sleep and health, biorhythms, metabolic responses, and athletic performance [27-29]. Correlation studies on exercise and sleep conditions including the influence of exercise intensity and hours on sleep [30], sleep disorders and performance capability [31-32], and the influence of melatonin on sleep have been conducted [33-34].
With regard to the role of melatonin in exercise and sleep, exercise late at night is reported to significantly decrease melatonin levels during sleep [35]. This result suggests that physical activities in improper time could cause sleep disorders through influencing melatonin level.
Sunlight is often considered as either beneficial or poisonous. Outside of ultraviolet rays, the visible light spectrum is generally considered to be poisonous. But a reasonable amount can be beneficial. Living in a sunny house helps healthy, bright, cheerful, and energetic life. Sunlight largely and variously influences health. Cycling adenosine monophosphate (cAMP) gets released by the stimulus of adrenocortical hormones due to intake of coffee, tea, chocolate, or stress. Excessive cAMP release accelerates energy release to reach excitement condition that leads us to be nervous and unsettled. However, sunlight exposure decreases cAMP, leading to peaceful and settled conditions [53]. Several studies have reported that sun exposure could improve the quality and state of the sleep as well as the sleep patterns, therefore improving the quality of life [36-38]. Although many studies have studied the correlation between sunlight exposure and sleep or sunlight exposure and exercise, studies on sunlight exposure and exercise on sleep are insufficient. Therefore, the objectives of the present study were to determine the effect of sun exposure and aerobic exercise on quality of sleep and investigate sleep-related hormonal responses in male university students who suffered from unemployment and stayed indoors for long hours.
METHODS
Object of study
Subjects were composed of male university students in their 20s who had no physical, medical, or sleep disorders. All subjects gave consent to this study.
They were divided into 4 four groups: sun exposure for 30 min (EG1), aerobic exercise with walking and jogging (EG2), aerobic exercise group with sun exposure (EG3), no exercise and no sun exposure control (EG4). Each group was assigned 10 subjects to compare and analyze changes in the quality of sleep and sleep-related responses according to sun exposure and exercise. Subjects had height of 175.8 ± 5.69 cm and weight of 73.2 ± 7.55 kg.
Sun exposure and exercise protocol
A total of 6 weeks of sun exposure and exercise were conducted to the same subject. Each experiment was conducted at 1-week interval to wash out the effect of previous experiment. A high-fat and high-protein diet was maintained for three meals a day without alcohol consumption. Diets of subjects were recorded prior to initial blood collection. Subjects were told to maintain similar diet prior to each blood collection after each experiment. Sun exposure was conducted by sitting outside for 30 minutes from 11 to 11:30 am to avoid excessively hot sunlight [39]. The average temperature was about 20℃. The average humidity was 60~70%. Aerobic exercises such as walking or running were performed. The exercise protocol consisted of five times of exercise session per week. Each exercise session contained 5 to 10 minute indoor stretching prior to and post exercise. Main exercise session was 30 minutes. Exercise intensity was measured by a wireless heart rate monitor (polar system). The intensity for each individual was moderate (50~60% of the maximum heart rate/HRmax) calculated with Karvonen formula by substituting target heart rate.
Surveys
Survey of the present study was conducted one time prior to each experiment in the morning of the day after each experiment. The questionnaire included simple personal information, questions about sleep pattern during weekdays (bedtime, wake-up time, and sleep onset latency), and Pittsburgh sleep quality index (PSQI) developed by Buysse et al. [40]. PSQI, a tool used to measure the quality of sleep, contains a total of 24 questions. The first part contains 19 questions to self-evaluate sleep. The second part contains five questions for cohabitant to confirm subject’s state of sleep. The present study used the first 19 questions of PSQI translated in Korean [41-42]. The 19 questions were divided into 7 subcategories: quality of sleep, sleep onset latency, amount of sleep, sleep efficiency, sleep disturbance, use of sleeping pills, and daytime impairment. The sum of each subcategory became the overall index for sleep quality evaluation. The higher the grade was, the lower the quality of sleep was. The credibility of this tool (Cronbach’s α) was 0.61.
Blood collection and analysis
Each blood collection per experiment was conducted in the morning after 8-hour fasting for a 5-day experiment per week. Blood was collected from the median cubital vein. A total of 10 ml blood was collected during each blood collection. Collected blood was stored at -80~-70℃ after serum separation following 5-minute centrifugation at 3,000 rpm. Stored blood was sent to a clinical center for analysis. Melatonin was protected from light and analyzed by radioimmunoassay using γ-counter (Cobra Qunatum, Packard Instruments Company, Meriden, USA). Cortisol was analyzed by radioimmunoassay using double antibody procedure (Coat-A-Count kit, diagnostic products corporation, Los Angeles, CA, USA). Catecholamine was analyzed by HPLCECD system (AS4000).
Statistics
Statistical analysis was conducted by using One-way ANOVA for comparison of the sleep satisfaction following sun exposure and/or exercise and of the sleep-related responses. All data were analyzed by SPSS ver. 18.0 to calculate the mean and the standard deviation of each measurement item. Statistical significance was set at p < 0.05.
RESULTS
The present study conducted experiments by dividing groups into sun exposure group (EG1), exercise group (EG 2), exercise group with sun exposure (EG3), and control group (EG 4) to compare and analyze the quality of sleep and sleep-related responses depending on sun exposure during exercise. The results on the quality of sleep (PSQI) and sleep-related responses (blood level of melatonin, cortisol, epinephrine, and norepinephrine) are summarized in the following:
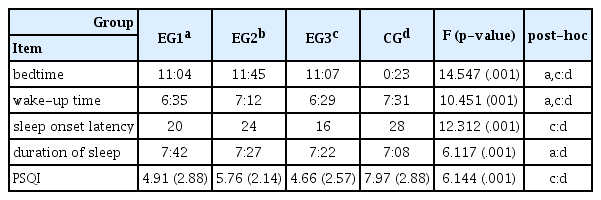
Effect of exercise with sun exposure on sleep behavior and quality of sleep
Effect of exercise with sun exposure on sleep behavior and quality of sleep was summarized in Table 1.
In Group 1, the bedtime and wake-up time were 11:04 pm and 06:35 am, respectively. It took them 20 minutes to fall asleep. The duration of sleep was 7 hours and 45 minutes. PSQI of group 1 was 4.91. In group 2, the bedtime and wake-up time were 11:45 pm and 07:12 am, respectively. It took this group 24 minutes to fall asleep. The duration of sleep of group 2 was 7 hours and 27 minutes. Their PSQI was 5.76. In group 3, the bedtime and wake-up time were 11:07 pm and 06:29, respectively. It took this group 16 minutes to fall asleep. The duration of sleep for this group was 7 hours and 8 minutes. Their PSQI was 4.66. In the control group, the bedtime and wake-up time were 00:23 am and 07:31 am. It took this group 28 minutes to fall asleep. The duration of sleep of this group was 7 hours and 8 minutes. Their PSQI was 7.97.
Effect of exercise with sun light exposure on sleep-related responses
Effect of exercise with sun light exposure on sleep-related responses was summarized in Table 2.
Changes in melatonin were 12.8 ± 2.83, 11.19 ± 1.28, 14.55 ± 2.53, and 9.45 ± 2.75 pg/ml in group 1, group 2, group 3, and control group, respectively. Melatonin level of group 3 was significantly (p < 0.05) higher than that of the control group. Cortisol levels in group 1, group 2, group 3, and control group were 11.13 ± 2.76, 13.56 ± 3.13, 12.56 ± 2.57, and 14.81 ± 2.63 μg/ml, respectively. Group 1 had lower cortisol level than that of the control group. Epinephrine levels in group 1, group 2, group 3, and control group were 28.5 ± 12.57, 35.28 ± 13.36, 30.75 ± 12.31, and 43.19 ± 15.36 pg/ml, respectively. Group 1 had significantly (p < 0.05) lower level of epinephrine than that of the control group. Norepinephrine levels in group 1, group 2, group 3, and control group were 106.16 ± 23.35, 121.49 ± 14.67, 109.14 ± 15.51, and 132.76 ± 21.19 pg/ml, respectively. Group 1 had significantly (p < 0.05) lower norepinephrine level than the control group (Table 2).
DISCUSSION
Healthy sleep affects physical and mental recovery, memory, learning, growth, and active longevity. The proper average amount of sleep is known to be 8 hours. In regard to sleep, human body has a circadian rhythm following a 24-hour cycle. Irregular sleep and sleep disorders can cause anxiety, stress, overwork, excessive exercise, jet lag, and endocrine disorders in the pineal gland, which can result in insomnia or hypersomnia. Sleep disorders of the elderly are considered as warnings for future degenerative diseases [4]. Melatonin is a sleep inducing hormones. It has a secretion cycle related to sleep. Melatonin is secreted the most at night. It is rarely secreted during the day. It is closely related to the initiation of deep sleep and the maintenance of a good quality of sleep [21].
Sun exposure improves the quality of sleep, state of sleep, and sleep patterns. Furthermore, it improves the quality of life [36-38]. Previous studies on the correlation between sunlight and sleep reported that changes in melatonin secretion improved the quality of sleep [36]. For the elderly with low melatonin secretion, sun exposure improved their sleep [43].
Even though risk factors of obesity are generally known as family history, eating habits, amount of exercise, and other lifestyle-related habits through previous studies. Recently, discussions on the effects of duration and quality of sleep on obesity are being actively progressed [44]. Hauri [24] reported that exercise can enhance the quality of sleep. Metabolic responses [45], athletic performance [46-47], and health [28-29] with regard to biorhythms have been studied. Correlation between exercise and sleep conditions such as exercise intensity and hour affecting sleep [30], sleep disorders and athletic performance [31], excessive exercise affecting sleep [30,48,49], athletic performance affected by sleep deprivation [31,50], and melatonin affecting sleep [33-34] have been reported. The present study showed that the sun exposure group and exercise group with sun exposure had the earliest bedtime and wake-up time. The exercise group with sun exposure presented the shortest sleep onset latency. Meanwhile, the control group showed the latest bedtime and wake-up time and the longest hours to reach deep sleep with shorter duration of sleep. The quality of sleep in the control group was low. In addition, melatonin secretion got lower in the order of exercise group with sun exposure and the sun exposure group without exercise. The sun exposure group showed low level of cortisol, epinephrine, and norepinephrine compared to the control group. Sleep restores physical functions and mental health beyond simple recovery. Sleep plays an important role in arranging and memorizing newly acquired information. Therefore, adequate amount of sleep is important for physical and mental health as well as task performance. However, for university students, they tend to be the evening type which is also observed at adolescence [51]. In other words, they are frequently active late at night due to task performance for educational purpose or social activities [52]. Meanwhile, they usually have difficulties to maintain healthy sleep habits due to the lack of sleep caused by lecture schedule or other social requirement that starts early in the morning. They have decreased quality of sleep following irregular sleep-wake cycle. Besides, learning activity in libraries for future job seeking and day and night part time job to solve financial problems can result in their lack of sleep and reduced physical activities. Therefore, university students are more vulnerable to sleep problems related to decreased physical activities. Therefore, it is important for them to grasp sleep patterns and physical activities. The present study showed that exercise group with sun exposure and sun exposure group without exercise positively affected sleep patterns and the quality of sleep. Other than merely accepting the finding that lack of sleep and reduced physical activities were caused by stress from financial status and job seeking of university students, it is important to educate and encourage university students to have enough time to exercise under sunlight for at least 30 minutes a day. This is important for their healthy sleep which affects academic performance and social relationships.
CONCLUSION
By comparing the quality of sleep, the order of bedtime of subjects from early to late was: the sun exposure group > the exercise group with sun exposure > the exercise group > the control group. The control group had significantly later bedtime than that of the sun exposure group or the exercise group with sun exposure. The order of wake-up time from early to late was: the exercise group with sun exposure > sun exposure group > the exercise group > the control group. Sleep onset duration got longer in the control group compared to the sun exposure group or the exercise group with sun exposure. The control group also showed lower quality of sleep. The duration of sleep in the order of short to long was: control group < exercise group with sun exposure < exercise group < sun exposure group.
In comparison of sleep-related responses, melatonin level was significantly higher in the exercise group with sun exposure than in the control group. The level of cortisol, epinephrine, and norepinephrine in the order low to higher was: the sun exposure group < the exercise group with sun exposure < exercise group < the control group. However, these levels were not significantly different. Based on these results, we concluded that both sun exposure group and exercise group with sun exposure positively affected sleep-related hormonal responses, showing good sleep habits and high quality of sleep.
Acknowledgements
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (NRF-2011-35CG00265).