Effects of low calorie diet-induced weight loss on post-exercise heart rate recovery in obese men
Article information
Abstract
[Purpose]
Heart Rate Recovery (HRR) after maximum exercise is a reactivation function of vagus nerve and an independent risk factor that predicts cardiovascular disease and mortality. Weight loss obtained through dietary programs has been employed as a therapy to reduce risks of cardiovascular disease and obesity.
[Methods]
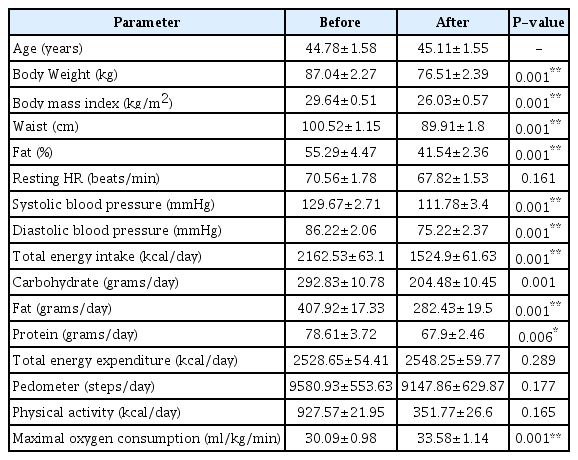
Eighteen subjects of middle aged obese men (age 44.8 ± 1.6 yrs, BMI 29.7 ± 0.5 kg/m2) were selected for this study. As a weight loss direction, the nutritional direction of low-calorie diet mainly consisted of carbohydrate, protein, and fat has been conducted for 3 months. Blood pressure was measured after overnight fasting, and blood samples were collected from the antecubital vein before and after weight loss program. All the pre- and post-exercise ‘HRR decay constant’s were assessed by using values of HRR (heart recovery rate; 2 minutes) and HR measured after reached to the maximal oxygen uptake (VO2max) exploited the bicycle ergometer.
[Results]
After the completion of weight loss program, body weight and BMI were significantly decreased, but the Heart Rate (HR) after maximum exercise and in steady state were not changed significantly (p > 0.05). The post-exercise HRR after the weight loss did not show significant changes in perspectives of 30 seconds (-16.6 ± 2.3 to -20.2 ± 2.1 beats/min, p > 0.05) and 60 seconds (-33.5 ± 3.4 to -34.6 ± 2.8 beats/min, p > 0.05) respectively but in perspectives of 90 seconds (-40.9 ± 2.6 to -48.1 ± 3.1 beats/min, p < 0.05) and 120 seconds (-48.6 ± 2.6 to -54.3 ± 3.5 beats/min, p < 0.05), they were decreased significantly. Pre-’HRR decay constant’s of 0.294 ± 0.02 %/second were significantly increased to post-values of 0.342 ± 0.03 %/second (p = 0.026). Changes in ‘HRR decay constant’ were significantly correlated with changes in blood glucose (r = -0.471, p < 0.05) and maximal oxygen consumption (VO2max, r = 0.505, p < 0.05) respectively.
[Conclusions]
The low-calorie diet directed to obese middle aged men for 3 months significantly improved the HRR after maximum exercise, and this improvement in cardiovascular autonomic nerve system was estimated to be involved with improvements in blood glucose and maximal oxygen consumption.
INTRODUCTION
Heart Rate Recovery (HRR) after maximum exercise carried out by the balance of vagus nerves and sympathetic nerves has been known as a reactivation function of vagus nerve and as an independent risk factor that predicts cardiovascular disease and mortality [1-3]. And this is also used as an indicator to predict death rate from cardiovascular disease of male patients having diabetes [4] and to predict the coronary artery disease of heart of people having familial hypercholesterolemia [5]. The significant low post-maximum-exercise HRR of people obese and having risk factors in cardio vascular metabolism compared to normal people has been identified through several studies [6,7]. That is, the low post- maximum-exercise HRR of obese people who excessively accumulated the body fat implies the low recovery rate of autonomic nerves after experiencing the exercise stress.
There are reports assessed the HRR after maximum exercise from studies conducted to improve the low HRR of obese people through weight loss. For instance, according to results of recent study of ‘Look AHEAD (Action for Health in Diabetes)’ study group upon large scaled group of 4,503 Type II diabetes patients conducted dietary control and exercise therapies together for 1 year, the HRRs after maximum exercise along with weight losses were reported to be improved significantly [8]. Wasmund et al. (2011) [9] also reported significant decreases of HRR after maximum exercise (average 13 beats/min) and heart rate in steady state attributed to the weight loss from gastrectomy. Furthermore, Brinkworth et al. (2006) [10] reported that the HRRs of obese men having risk factors of metabolic syndrome were significantly decreased by weight loss through dietary control. And the HRR of obese and over-weighted woman having polycystic ovary syndrome accompanied insulin resistance was reported to be significantly decreased after 10 weeks of intervention experiment carried out through the low calorie diet of 30% dietary restriction [11]. Thus the weight loss due to gastric resection or dietary restriction decreases the HRR significantly. Significant decrease in post-exercise HRR due to weight loss was reported however, views upon factors involved in changes of HRR through such dietary restriction were not presented.
Meanwhile, the maximal oxygen consumption (VO2max) has been emphasized as a strong indicator predicting the cardiovascular death rate of men [12,13]. In the recent context of concurrent decrease in cardiopulmonary physical strength and HRR [14], the relationship between physical strength improvement due to weight loss through dietary restriction and HRR of obese men should be investigated.
Differences of HR between in perspectives of 1 minute or 2 minutes after maximum exercise were simply adopted for the actual assessment of HRR but Kim et al. (2009) [15] presented the ‘HRR decay constant’ as a new indicator for the assessment of HRR after exercises. However, studies on effects of weight loss due to dietary restriction upon ‘HRR decay constant’ are yet to be conducted.
As mentioned before, classical HRR was employed in prior studies for the assessment of people having diverse diseases (e.g., metabolic syndrome, polycystic ovarian syndrome, diabetes, familial hypercholesterolemia etc.) thus information upon factors involved in such changes particularly upon changes in physical strength is not sufficient. Therefore, this study was designed to examine the effects of diet induced weight loss on ‘HRR decay constant’ in obese men who were free from medication for diseases, and to assess its relevance to cardiorespiratory functional capacity and blood constituents as a factor involved with changes in heart rate after maximum exercise.
METHODS
Subjects and data preparation
Subjects of this study selected among people currently living in ‘T’ city were recruited by telephone counseling and posted handbills. People having orthopedic, cardiac, and/or metabolic disorders who would be inadequate for the exercise of this study were excluded from the subjects. Particularly, people under medication (for hypertension, diabetes, and hyperlipidemia etc.) were excluded except selected 18 normal obese male subjects.
Anthropometry
Each subject’s measurement was measured at least 10 hours overnight fasted states. Heights of subjects were measured by digital instrument (TBF-215; Tanita, Tokyo, Japan) of which precision was 0.1cm, and subjects’ weights on barefoot with inner wear were measured by scale (TBF-215; Tanita, Tokyo, Japan) of which precision was 0.01kg. The BMI was obtained through dividing each value of weight by corresponding squared value of each height. Waist line was measured at the position of bellybutton. Average values obtained from two consecutive measurements were selected as measured values.
Maximal aerobic capacity
To measure individual maximal oxygen uptake of each participant, at least 5 minutes warming up mainly consisted of the stretching of quadriceps muscle of thigh, hamstring muscle, and gastrocnemius was practiced at the fitness center.
Using the bicycle ergometer (818E; Monark, Stockholm, Sweden), the cardiorespiratory functional capacity was measured as in the report of a previous study [15]. After completing the 2 minutes warm up at 0W, the exercise was started at 15W. Load of 15W was gradually added at every minute to the limit of each subject’s capacity.
Respiration and breathed gas were measured through the automated gas exchanging system (Oxycon α system; Mijnhardt, Breda, The Netherlands). Heart rates during each exercise and rest were measured by ECG monitor (Dynascope; Fukuda-denshi, Tokyo, Japan). The breathing gas analyzer automa-tically made the point encountering two lines. Obtained maximal amount of oxygen consumption lasted over 30 seconds was determined to be VO2peak.
Exercise tolerance test was terminated in cases of 1) The rating of perceived exertion over 18 (Borg’s scale), 2) The HR over 90% of maximal HR estimated by age or the subjects were extremely exhausted unable to pedaling, and 3) Serious arrhythmia or excessive anomaly in ST segment (over 1mm horizontal or downward, descended ST segment). Based on these criteria, cases manifested ischemic responses from exercise or having chronic disorders (not reached to the level of 80% of each individual’s age adjusted maximal HR) were excluded from the measurements.
Measurement of blood test
All bloods were analyzed from antecubital vein after at least 12 hours of overnight fasting. Bloods collected in 8 ml tube contained thrombin and heparin neutralizer (Venoject Ⅱ, TERUMO, Japan) were immediately centrifuged for 10 minutes at 3000 rpm under temperature of 4℃. Blood insulin was measured by using commercial enzymatic immunoassay kit (Wako Pure Chemicals. Ltd, Japan), and the triglyceride and cholesterol were measured by enzymatic assay employed the commercial kit (Wako Pure Chemicals. Ltd, Japan).
Time course of heart rate after maximal exercise
Heart rate after exercise was measured from participants on chairs sat immediately after terminating exercise (reached to respective maximal aerobic capacity) using method presented in prior study [15]; and heart rates during exercise and rest were continuously measured using the ECG monitor. The heart rate after exercise was measured at every 30 seconds for 2 minutes from the heart rate of maximum exercise. ‘HRR decay constant’ was assessed using the slope of least-squares regression line of natural logarithm of each measured value of heart rate presented as new measurement method in the prior study [15].
Dietary records
The participants were directed not to change each exercise habit during weight loss period of dietary restriction to identify the sole dietary effects. Classroom for dietary therapy lessons was opened once in a week and the 90 minutes programs for each classroom were lasted for 3 months. The dietary restriction program for weight loss employed the previously reported method i.e. the four-group point method [16]. The foods were classified into 4 groups (Group 1: eggs, dairy products etc.; Group 2: meats, fishes, soy milk products etc.; Group 3: greens, vegetables, seaweeds, fruits etc. Group 4: milk fat, sugar or sweeteners etc.), and participants were encouraged to take total 1680 kcal daily by taking 560 kcal at each meal where 80 kcal was counted as 1 nutritional point. Participants recorded ingested foods every week in amounts of carbohydrate, fat, and protein and these records were analyzed by professional dietician.
Data analysis
All data presented in this study were calculated by statistical package of SPSS Version 20 to produce descriptive statistics (mean ± standard error). Analyses between variables before and after respective measurement employed the paired-t test. To verify the relevance of heart rate decrease coefficient with each variable the Pearson's correlation coefficient was extracted. A two-way analysis of variance (ANOVA) with repeated measures with post hoc Bonferroni was used to analyze the time course in post exercise HR. Level of all statistical significance was set as α = .05.
RESULTS
Examination of exercise loads
No participants appealed chest pains or pains in hip or knees during gradual maximum exercise tolerance test through bicycle ergometer and abnormal wave mode in electrocardiogram (e.g. ST segment elevation or depression) or abnormal heart beat (e.g. decrease in HR despite of increase in exercise intensity etc.) were not observed. All obese male subjects participated to the test showed BMIs over 25 kg/m2 and the basic physical characteristics of voluntary subjects are summarized in Table 1.
Changes in blood parameters after low-calorie diet program
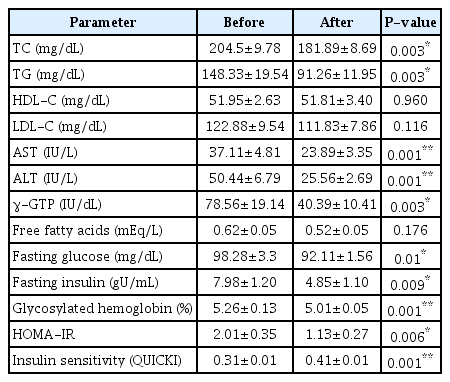
Changes in blood constituents after weight loss compared to those before weight loss are summarized in Table 2. Blood lipid, TC, and TG were all decreased significantly (p < 0.05). Serum enzymes, AST, ALT, and γ-GTP as parameter of liver function, were also significantly decreased respectively. Regarding changes in variables related with blood glucose, the HOMA-IR which is an indicator of insulin resistance was significantly decreased along with significant decreases of blood glucose, insulin, and HbA1c (p < 0.05).
Variables related with HRR and heart rate after weight loss
Before the experimental intervention, changes in HRR right after each maximum exercise manifested significant negative correlation with the heart rate in steady state (r = -0.545, p < 0.19). Through the low calorie diet weight loss program conducted for 3 months the body weights and BMIs were significantly decreased (p < 0.05). However, heart rates right after maximum exercises and in steady states did not show significant differences respectively (p > 0.05).
Temporal changes in HRR after maximum exercise
Fig. 1 Illustrates the temporal progress of heart rate measured at each interval of 30 seconds in 2 minutes after maximum exercise.
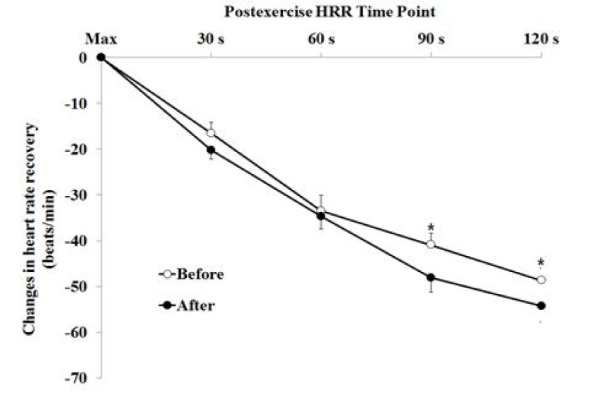
Post-exercise heart rate recovery at each time before and after the weight loss achieved in 12-weeks. The graph illustrates the statistics of mean ± standard error. * Significantly different, p < 0.05
After the weight loss, post-exercise HRRs measured at 30 seconds (-16.6 ± 2.3 to -20.2 ± 2.1 beats/min), at 60 seconds (-33.5 ± 3.4 to -34.6 ± 2.8 beats/min), at 90 seconds (-40.9 ± 2.6 to -48.1 ± 3.1 beats/min), and at 120 seconds (-48.6 ± 2.6 to -54.3 ± 3.5 beats/min) were all decreased respectively.
Statistical significance at each time appeared as follows: after the exercise (p > 0.05), 30 seconds later after exercise (p > 0.05), 60 seconds later after exercise (p < 0.05), 90 seconds later after exercise (p < 0.05), and 120 seconds later after exercise (p < 0.05)
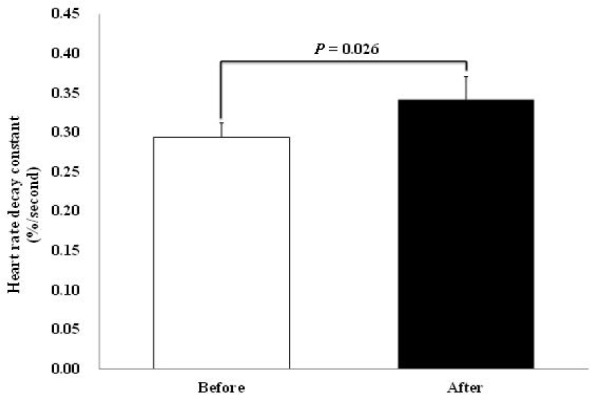
Fig. 2 Illustrates the changes in ‘HRR decay constant’ made through the weight loss. The value increased significantly from the one measured at pre-test (0.294 ± 0.02) to the value measured at post-test (0.342 ± 0.03 %/second) (p = 0.026).
Changes in ‘HRR decay constant’
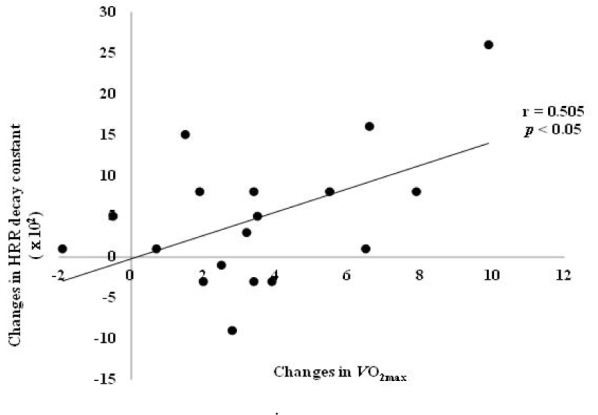
Fig. 3 Illustrates significant correlations of changes in ‘HRR decay constant’ resulted from low calorie diet with changes in VO2max (r = 0.505, p < 0.05) and changes in fasting blood glucose (r = -0.471, p < 0.05). However, relevance with other blood constituents and blood lipid was not manifested (p > 0.05).
DISCUSSIONS
The delayed recovery of heart rate after exercise is related with autonomic nerve system of the heart and has been used as an indicator implying improved cardiovascular disease. As described before, the recovery of slow heart rate after maximum exercise was reported to be related with cardiovascular death rate increase [1] thus the recovery rate of stressed heart is quite important. This study was designed to examine changes in the independent factor (the ‘HRR decay constant’) related with cardiovascular death rate and was introduced as a new indicator for the assessment of recovery rate of heart rate to be changed by weight loss induced by low calorie diet and, to investigate variables related with recovery rate of heart rate.
The results of the study showed that the weight loss of obese middle aged men induced by low calorie diet significantly decreased the heart rate after maximum exercise and the fasting blood glucose and cardiopulmonary physical strength were appeared to be involved in such decrease in HR.
In the recent study conducted to find the relevance of metabolism with heart rate recovery after maximum exercise [6], the decreased recovery of heart rate of juveniles after maximum exercise practiced by bicycle ergometer was reported to be related with the waist circumstance as the obesity indicator and was inversely correlated with cardiometabolic risk factors such as blood glucose, cholesterol, neutral fat, and/or blood pressure. Deniz et al. (2007) [17] compared the heart rate recovery for 1 minute after maximum exercise in groups of males aged in their 20s who were classified into groups by the presence of metabolic syndrome, and reported the significantly decreased HRR in the group having metabolic syndrome.
For results of pathophysiological studies, Karjalainen et al. (2012) [18] examined the recovery rate of heart rate after maximum exercise from patients grouped by the presence of diabetes and coronary heart disease and reported that the patients of coronary heart disease having diabetes together showed significant low recovery rate of heart rate compared to other patients without diabetes, and interestingly, the recovery rate of heart rate was also reported to be related with obesity and physical activity more closely than other complications or terms exposed to diabetes. Tigen et al. (2009) [19] reported that the reduced recovery rate of heart rate of obese people after maximum exercise was related with left ventricular systolic dysfunction along with the decrease in motor function. Results of such studies lead us to the hypothesis suggesting that the dissipation of obesity through weight loss or through increased physical activity employed exercises might be related with the recovery rate of heart rate.
Actually, the exercise training improves the heart rate recovery rate after maximum exercise. Kim et al. (2009) [15] examined the ‘HRR decay constant’ which were introduced as a new substitute marker for heart rate recovery after maximum exercise carried out for 3 months in groups of obese men classified by the presence of metabolic syndrome, and found the heart rate of obese men free from metabolic syndrome after maximum exercise practiced by bicycle ergometer were not significantly changed along with significant decrease in weights (average 3.0 kg), and on the contrary, reported that the obese men belonged to the group of metabolic syndrome manifested the significant decrease in heart rate after maximum exercise (0.31 ± 0.02 %/sec to 0.35 ± 0.02 %/sec, p > 0.05) along with the decreases in weights. This implies that changes in autonomic nerve system of heart might be dependent more upon metabolic characteristics of obese people than the weight loss induced by exercise program. There are prior studies investigated the influences of weight loss of obese people upon changes in heart rate after maximum exercise. Brinkworth et al. (2006) [10] reported results from the study on heart rate recovery of obese men after maximum exercise conducted for 3 months along with low calorie diet where the heart rate for 1 minute after maximum exercise practiced by treadmill after 12 weeks of weight loss program limited 30% of energy uptake was significantly decreased and this decrease was significantly related with the body weight loss (r = -0.41, p = 0.008). And the significant relevance of weight loss with reduced blood glucose (r = 0.37, p < 0.01) was also reported [10]. Such results were accorded with results of this study. But different from results reported by Brinkworth et al., 2006 [10] the results of this study upon changes in recovery rate of heart rate after maximum exercise was presented by employing the new indicator, the ‘HRR decay constant’. Of course, in prior studies, the recovery rate of heart rate was measured at various time zones. For instance, maximum heart rates at 1 minute, 2 minutes, 3 minutes, 4 minutes, and/or 5 minutes were measured for the analysis of differences. But the ‘HRR decay constant’ in prior study was derived from the equation reflecting the decrease in heart rate measured at the most frequent time zone of 2 minutes.
In this study, the relevance of maximal oxygen consumption was presented to explore factors involved with changes in post-exercise heart rate. Changes in heart rate after maximum exercise reflecting the function of vagus nerves in cardiovascular autonomic nerve system and the maximal oxygen consumption are related with cardiovascular risks respectively, and are the strong indicators predicting death rates.
These factors suggest the involved relevance to changes made through weight loss. In this study, the weight loss achieved through dietary restriction manifested the significant relevance with ‘HRR decay constant’ and changes in maximum oxygen consumption. However, changes in maximal oxygen consumption along with actual dietary restriction were mostly due to the reduced amount of fat. Though they were not identified in this study, reviews on the relevance of changes in physical composition such as fats and muscle mass etc. to ‘HRR decay constant’ might be needed in further studies. Recent study [20] investigated obese women before menopause who improved life styles without dietary restriction for 3 months reported that the recovery of heart beat after maximum exercise showed the decrease in perspective of 60 seconds after the maximum walking exercise on treadmill (from 21.3 ± 6.2 to 27.8 ± 10.2 beats/min, p < 0.05). Such results differ from results of changes in heart rate (-33.5 ± 3.4 to -34.6 ± 2.8 beats/min, p > 0.05) measured in perspective of 60 seconds after maximum exercise conducted in this study. Actually, this comes from the difference in test protocols. Protocols related with finding heart rate recovery normally use treadmill or bicycle ergometer.
Though differences in maximal heart rate may be appeared in the same period of times as mentioned before but influences of each training and dietary restriction upon HR would be different from each other. And physiological characteristics of subjects could also produce different results. Particularly, in cases of measuring temporal heart rates right after aerobic exercises from supine and/or sitting positions in the examination of exercise tolerance test showed different responses in HRR respectively [21]. That is, different interpretations might be obtained along with different cool-down protocols.
In deriving conclusions of this study, there were limitations as follows: First, the autonomic nerve system of heart was not directly measured in this study. However, as it was identified in prior studies, the recovery of heart rate after maximum exercise reflects the autonomic nerve system of heart [22,2]. For the second, the heart rate after maximum exercise defined in the protocol was an assessment upon the recovery period of 2 minutes. Prior studies employed test protocols defined various time zones such as 1 minute, 2 minutes, or 5 minutes for the assessment. But the recovery of heart rate measured in perspective of 2 minutes after maximum exercise is typically applied for the assessment [8,15]. And for the third one, all subjects of this study were men. Difference in recovery of heart rate after maximum exercise exists between [23].
Therefore, the sex of subjects was limited to male to explore factors related with heart rate recovery after maximum exercises practiced along with each weight loss. Finally, the respective cardiorespiratory physical strength or past career of physical exercises might have been involved. Thus further studies considered multi-factors including involvement of diverse variables along with differences in sexes or heart rate measuring positions etc. would be required.
CONCLUSIONS
The influence of low calorie diet weight loss program for obese men upon heart rate recovery after maximum exercise was examined and following conclusions were obtained. First, the recovery of heart rate after maximum exercise practiced by bicycle ergometer along with the weight loss program of dietary restriction showed significant decrease in cases measured values in perspective of 90 seconds and 120 seconds. Second, the ‘HRR decay constant’s of obese men participated to the low calorie diet weight loss program were increased significantly. Third, the fasting blood glucose and changes in cardiopulmonary physical strength were identified to be the factors involved with such changes in ‘HRR decay constant’ respectively.
In short, the weight loss of obese men achieved through low calorie diet program was assumed to have brought the improvement in blood glucose and cardiopulmonary function along with enhanced heart autonomic nerve regulation.