Exercise Training suppresses vascular fibrosis in aging obesity induced rats
Article information
Abstract
[Purpose]
The aim of this study was to investigate the effects of exercise training (ET) on vascular fibrosis in aging model rats with diet-induced obesity.
[Methods]
Twenty-four male Sprague-Dawley rats were divided into 3 groups: Aging control (A-C), A-C with high fat diet (AHF), AHF with ET (AHF + ET). Aging was induced by D-galactose (D-gal) and obesity was induced by HFD (60% fat) for 9 weeks. The experimental rats performed swimming (60 min/day, 5 days/week) for 8 weeks. All rat aorta samples were harvested for RT-PCR and morphologic analyses.
[Results]
The exercise training significantly decreased levels of AT-1, TGF-ß and Coll-1 gene expression compared to AHF group. The AHF + ET group showed a reduced collagen accumulation in the aorta media compared to AHF group.
[Conclusion]
These results suggest that ET could protect the aging obesity aorta against down-regulation of fibrotic factors (AT-1, TGF-ß and Coll-1 gene) and fibrosis by inhibition of collagen accumulation in the aorta media.
INTRODUCTION
Metabolic syndrome (MS) has been globally increasing and results in obesity, insulin resistance, dyslipidemia, and hypertension [1]. It results in an increased risk of cardiovascular diseases (CVD) [2]. Atherosclerosis is commonly caused by the development of CVD with aging and understood to be associated with vascular fibrosis. Vascular fibrosis is characterized by a reduced lumen diameter and arterial wall thickening and associated with many clinical diseases and pathological progresses [3]. Many studies [3-5] have reported that the expression of main risk factors (angiotensin II (Ang)), transforming growth factor beta (TGF-ß), collagen and fibronectin in extracellular matrix (ECM) for vascular fibrosis was increased in vascular tissue.
Ang II, a vasoconstrictor produced by cardiovascular system, is a vital component of renin-angiotensin system that affects blood pressure and is also linked to obesity via fat and muscle metabolism [4]. Moreover, Ang II binds to the angiotensin receptor (AT)-1, and activation of AT-1 is present in most of the Ang II-mediated cardiovascular responses (vasoconstriction, hypertrophy, fibrosis, inflammation) [3].
TGF-ß is involved in regulating the accumulation of ECM, and vascular remodeling via activation of the production of several factors including connective tissue growth factor and fibroblast growth factor [3].
Vascular fibrosis induced by accumulation of collagen type I (Coll-I) and collagen type III (Coll-III) can lead to atherosclerosis [3]. In addition, collagens in aging vessels are primary a function of reduced degradation and activated collagen synthesis and proliferation of fibroblasts [6,7].
There are several known risk factors that can lead to vascular fibrosis in arterial stiffening, atherosclerosis. Thus, one of the treatment options is to inhibit Ang II, TGF-ß and collagen expression in the artery in those CVD with aging, including vascular fibrosis [8].
Inactivity or sedentary lifestyle is one of the primary causes for the development of obesity, decreases cardiac function, and aggravates CAD [9]. It is widely known that inactivity appears to promote cardiovascular aging [10].
Exercise training (ET), particular aerobics exercise, effectively produced predictable changes in the body composition including muscles and fat fads. More importantly, ET has been known to improve cardiovascular function in human and experimental animals [11,12]. It is possible that ET in aging with MS increases the maximal cardiovascular work capacity by increasing the stroke volume and cardiac output [9], and it also may result in decreased accumulation of connective tissue such as collagen.
However, there is a lack of research in the investigation on the effects of ET on aging with obesity resulting in vascular fibrosis, especially in morphological phenomena in vivo. Analysis of AT-1, TGF-ß and Coll-1 gene expressions with ET have not been clearly identified in D-galactose (D-gal)-induced aging with high fat fed rats resulting in fibrosis.
The purpose of the present study was to evaluate the effects of ET on the vascular fibrosis in artery of rat with aging obesity induced by D-gal administration with high fat fed. This study also investigated the effects of ET on the expression of AT-1, TGF-ß and Coll-1 gene associated with fibrosis in artery of aging rat with MS.
METHODS
Animals and diet
24 male Sprague-Dawley (SD) rats (body weight 200-250 g, 2 month old) were provided by Orient Bio laboratory animal center (Seoul, Korea). All experimental rats were randomly assigned into three groups after 1 week of adaptation as following groups: Aging control (A-C), A-C with high fat diet (AHF), and AHF with exercise training (AHF + ET). Control groups were fed a normal diet with 10 kcal % fat (100000, Dyets Inc, Bethlehem, PA, USA) and AHF rats were fed a HFD with 60% kcal fat (100496, Dyets Inc) for 9 weeks. All experimental animals were given free access to food and water. Treatment started on 1 week after D-gal injection and high fat diet. Procedure related to animals and their care was conducted in accordance with international guidelines ‘Principles of Laboratory Animals Care’ (NIH publication no. 85-23, revised 1985).
The study protocol for all animal experiments was approved by the institutional Animal Care and Use Committee of Hanyang University (HY-IACUC-11-050).
Aging induced D-gal
Aging was induced by D-gal (Sigma ST. Louis, MO) melted in normal saline. In Aging group, D-gal injection (100 mg/kg) was intraperitoneally (IP) given in the morning (10:00 am) every day for 9 weeks. A-C group was only injected with normal saline in IP without D-gal [13].
Exercise training
The total 8-week ET was performed in swimming chamber (diameter: 150 cm, height: 70 cm) at 30-32℃ water temperature. The swim exercise was performed for 60 minutes (min) per day, 5 days per week for 8 weeks. The exercise time was gradually increased for a week (for 5 min initially and then lengthened by 10 min daily for total 60 min) to allow an adaptation at the exercise [12].
Tissue preparation
After the experimental period, all rats were fasted for 12 hours, and anesthetized with ketamine/xylazine mixture. The aorta was rapidly removed and washed in a phosphatebuffered solution (PBS). The samples were stored at -80℃ for RT-PCR. The tissue was fixed with 4% paraformaldehyde-0.1% glutaraldehyde in 0.1 M PBS for morphological analysis. After fixation, samples were dehydrated in ethanol and embedded in paraffin and cross sectioned into 5-6 µm thickness.
RT-PCR analysis
Total RNA was isolated from ventricle by using TRIzol (Invitrogen, Carlsbad, CA). RNA samples were reversetranscribed with SuperScript II (Gibco BRL, Rockville, MD) and mix of oligo dT and random primers in a 12 μl total reaction volume at 45℃ for 30 min. PCR was performed using the cDNA as a template. The PCR reaction using a Taq DNA polymerase (TAKARA) and pair of primers were designed using BLAST algorithms (GenBank). The primer sequences used and conditions of synthesis are provided in Table 1. PCR products were separated on 1.2% to 1.5% agarose gels using a T3000 Thermocycler (Biometra, Goettingen, Germany). The densities of each band were analyzed by gel documentation (Gel Doc 2000, Quantity One, Bio-Rad, Hercules, CA).
Masson’s trichrome stain for collagen
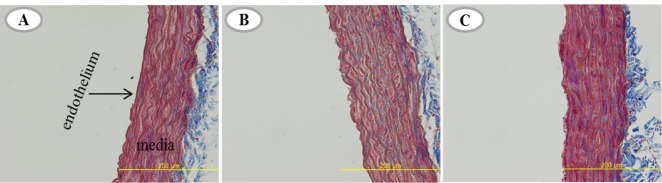
For the morphological analysis of collagen, tissues were used including an adaptation of the Masson’s trichrome technique. In this stain, collagen fibers are blue, other cells are bright red. Briefly, tissue sections were deparaffinized, and cleared. The sections were stained in Weigert iron hematoxylin for 10 min, and then washed in water, and then stained in Biebrich scarlet-acid fuchsin solution for 2 min. Samples were then immersed in aniline blue solution for 5 min, and then washed in water. After immersion in 0.5% glacial acetic acid solution for 3 min, they were dehydrated in ethanol, and then cleared in xylene. Finally, the slides were observed on a light microscope (Olympus U-LH 100HG, Tokyo, Japan).
Statistical analysis
Statistical analysis was performed using SPSS software (version 18.0). All data were presented as means ± standard error of mean (SEM) and analyzed by one-way ANOVA with a following Tukey. P < .05 was considered as statistically significant.
RESULTS
AT-1 gene in the aorta
As shown in Fig. 1, AT-1 gene in aorta was performed using RT-PCR (A) and graph (B). AT-1 gene were significantly increased (p < .001) in AHF group compared to control group, whereas it was significantly inhibited (p < .05) in the AHF + ET group compared to AHF group.
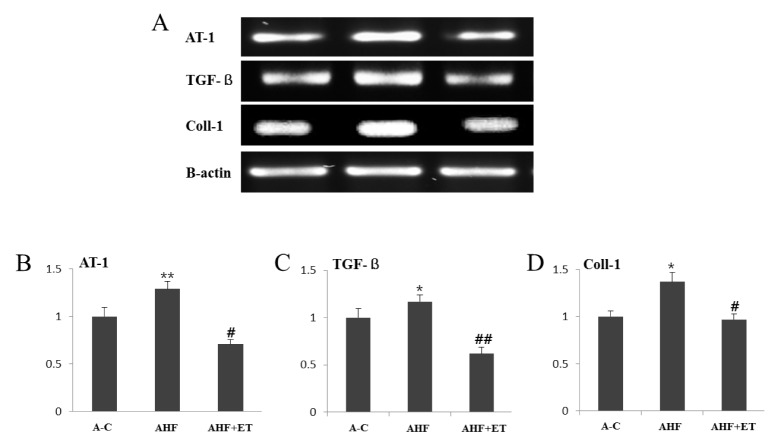
RT-PCR (A) and densitometric graphs (B, C, D) of AT-1, TGF-ß and Coll-1 in aorta. The gene expression of AT-1, TGF-ß and Coll-1 are significantly decreased in AHF + ET group compared to AHF group. The densitometric analysis of RT-PCR (B, C, D) are shown. Data are represented mean ± SEM (n = 6). A-C group (Aging control group), AHF group (A-C with high fat diet), AHF + ET group (AHF with ET: exercise training).
TGF-ß gene in the aorta
The expression level of TGF-ß gene was significantly elevated (p < .05) in AHF group compared to the other groups. In contrast, it was significantly inhibited (p < .001) in the AHF + ET compared to AHF group (see Fig. 1).
Coll-1 gene in the aorta
The expression level of Coll-1 gene was significantly increased (p < .05) in AHF group compared to the other groups. In contrast, it was significantly lowered (p < .05) in the AHF + ET group compared to AHF group (see Fig. 1).
Masson’s trichrome staining in the aorta
As shown in Fig. 2, we used Masson’s trichrome staining to visualize the fibrillar collagen. We found positive staining for collagen fiber (see blue) in aortic wall space and located in the media of all groups. Collagen positive staining in the AHF group was remarkably observed with the clear linear. However, AHF + ET group was substantially weak.
DISCUSSION
Aging obesity is known to not only promote the development of vascular disease but also to be associated with significantly lipid metabolism including body weight (BW) gain, obesity-related diseases such as heart diseases, hypertension, atherosclerosis, and type-2 diabetes mellitus [1,2,14]. Moreover, the incidences and numbers of hypertension are increased in the elderly [15], which is in part due to arterial fibrosis associated with remodeling [3-5]. Many risk factors for the arterial fibrosis can be treated by life-style changes such as regular exercise. The therapy for the fibrosis is effective and protective against the risk of CVD death [14]. The present study established a rat model of arterial fibrosis by feeding with HF and aging with D-gal. D-gal, oxidative stressor, is known to induce aging in chronic administration [13]. Previous studies used the biochemical method to induce aging by D-gal leding to atherosclerosis and tissue damage in experimental animals [13,16]. Vascular aging promotes the development of vascular diseases through an abnormal release of vasoactive factors and increases BW [14].
High fat (HF) diet is generally used by researchers to induce obesity, lipid accumulation and atherosclerosis vascular disease in rodent’s models [17]. Therefore, in this study, we used D-gal induced aging rat models with HF diet induced vascular fibrosis.
Although many studies have been conducted on the prevention of vascular fibrosis [17], it is still not understood yet how physical exercise improves the fibrosis in aging artery. Thus, we investigated the preventive effects of ET on fibrosis factor associated with age related MS.
Exercise in human and animal suppresses fat deposition and BW gain, and controls lipid profiles [18]. Exercise also reduces risk factors associated with CVD, and its anti-fibrosis effects have been demonstrated in the experimental studies [8].
In the present study, ET remarkably suppressed the expression of AT-1, TGF-ß and Coll-I gene in aorta. Interestingly, we found that ET has been effective in controlling fibrotic factors and was attenuated to visualize the collagen accumulation (Masson’s trichrome) in aorta than AHF group. It is known that ET elevates blood flow and shear stress to activate the factors related to vascular function and vascular elasticity. In addition, ET decreases the synthesis of vasoconstrictors [19] and increases the expression of vasodilatation [20], cardio output and plasma volume, and attenuates vascular resistance and Ang II protein expression [12].
Lan et al. [3] reported that Ang II, TGF-ß and Coll-1 in the aorta increased with vascular remodeling and can lead to vascular fibrosis including CVD. However, Kwak et al. [8] reported significantly decreased collagen with regular ET for 12 weeks. This study demonstrated that regular ET attenuated activation of AT-1 receptor, TGF-ß and Coll-I gene expression in the aging rat aorta with MS.
Ang II is known to induce vascular fibrosis and hypertension, leading to pathological sate cell growth/apoptosis of vascular cells, inflammatory responses and ECM remodeling [21]. It also promotes endothelial dysfunction and structural remodeling and acts through its binding to main specific receptor, AT-1. AT-1 implicated in cell growth and hypertrophy [3].
TGF-ß is expressed in cytokines and increased in ECM remodeling [22] and plays a critical role in ECM accumulation and vascular remodeling via activation the expression of other factors, including growth factor. Ang II and reactive oxygen stress have been known to stimulate TGF-ß up-regulation as a mediator of vascular fibrosis [3].
Coll-1 accumulation, advanced glycation end product related cross linking, and fibrosis with aging are progressive and associated with weaken cardiac contractility and CVD risk [8,23] These genes activate fibrotic connective tissue which impair cardiovascular function and lead to increased vascular fibrosis, therefore elevation the risk of CVD with aging obesity [23].
In the present study, we observed the vascular fibrosis by D-gal administration in the HF diet group. The AHF group showed the elevated AT-1, TGF-ß and Coll-I gene expression that was visualized with collagen positive staining in aorta media. Previous studies demonstrated that aging obesity induced abnormalities in endothelial cell size and shape, susceptibility to apoptosis, angiogenesis, and abnormal release of vasoactive factors [14].
Other data further indicated that fibrosis induced by D-gal administration with HF diet caused AT-1, TGF-ß and Coll-I gene expression increase which leads to pathological vascular remodeling or vascular apoptosis, thus producing matrix accumulation [3,14].
Therefore, we demonstrated that regular ET inhibited aging obesity induced-fibrosis. This anti-fibrotic effect of regular ET might be caused by inhibition of AT-1, TGF-ß and Coll-I gene expression in association with the reduced vascular wall remodeling and collagen accumulation in aorta.
We found that ET induced decreases in collagen fiber accumulation in aorta, shown by Masson’s trichrome techniques. This was the first study on the morphological effect of protective fibrosis in the aging obesity aorta.
Consequently, as a result of present study, regular ET should be one of the treatments to control fibrosis in aging obesity aorta. Further studies are needed to unravel the underlying molecular mechanism related to the anti-fibrotic effect of exercise.
Acknowledgements
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-358-2011-1-G00036).