The effects of a single bout pilates exercise on mRNA expression of bone metabolic cytokines in osteopenia women
Article information
Abstract
[Purpose]
The purpose of this study was to examine the effect of a single bout pilates exercise on mRNA expression of bone metabolic cytokines in elderly osteopenia women.
[Methods]
We selected 11 people of elderly osteopenia women and loaded a single bout pilates exercise about RPE 10-14 level. The blood samples were collected before, immediately after and 60 minute after pilates exercise, then examined calcium metabolic markers in serum and extracted peripheral blood mononuclear cell (PBMC) from whole blood and confirmed mRNA expression of bone metabolic cytokines from PBMC. To clarify the changes during exercise, we designed repeated measure ANOVA as the control group to perform blood sampling without exercise.
[Results]
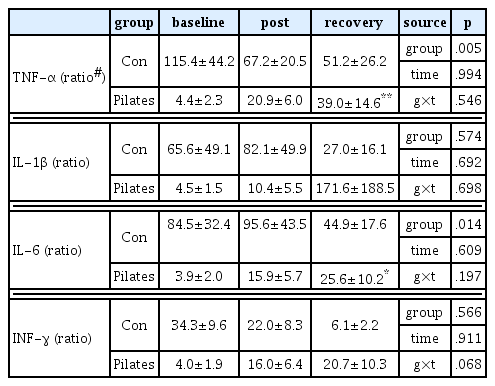
As a result, serum P showed significant interaction effect between group and time (p<.001), the pilates exercise group decreased about 9% at immediately after exercise and 13% during recovery after exercise (p<.05), while the control group showed a tendency to increase. Serum CK also showed a significant interaction between group and time (p<.05), the pilates group significantly increased at immediately after exercise and during recovery after exercise (p<.05) but the control group didn’t have changes. TNF-α and IL-6 mRNA expression in PBMC was significantly increased in the pilates group (p<.01, p<.05), although INF-γ mRNA expression didn’t show statistically significant difference, it tended to increase in the pilates group (NS).
[Conclusion]
These results suggested that a single bout pilates exercise of elderly osteopenia women cause hypophosphatemia with temporary muscle damage, and it leading high turnover bone metabolic state with to activate both of bone formation and bone resorption.
INTRODUCTION
Bone is an important organ to perform exercise by support the body and has various functions such as a store for calcium and other minerals which is essential for sustaining life, and hematopoietic action creating new blood [1]. In addition, bone is a highly active tissue in the body, which is sensitive to external mechanical stimuli as well as internal metabolic changes. So it occurs changes in bone mineral density (BMD) and bone metabolism. These changes in bone metabolism are influenced by the environmental factor such as gene [2] and nutrition [3], and the exercise do the most important role to increase and maintain bone mass in particular [4]. That is, the inactivity environment such as space flight [5] or weightlessness [6] stimulates to reduce bone mass, but it is known that regular exercise is suitable to maintain or increase bone mass [7]. Nuti et al. [8] also prove that even the period of growth and the adolescence, elderly group has a different form of exercise, exercise is effective on enhancing BMD. However, most of the research is usually focused on 'phenomena' that reveal a degree of changes in BMD as the effects of exercise, but it is lacking to study about ‘mechanism’ how changes are made by. That is, it isn’t clear yet whether BMD increased by increasing bone formation or by suppressing bone resorption even in BMD increasing by exercise. Thus, it plays an important role in the development of exercise science to clarify bone metabolism the differences between bone formation and bone resorption by exercise and to establish detailed mechanism about exercise effect in the cell or in the cell-to-cell. Exercise causes changes in bone mass by stimulating bone cells directly or indirectly, and especially they are created together in the bone marrow cells, immune cells as well as bone related cells. Thus, various control factors such as cytokines share information with bone and immune metabolism, bone formation cells, immune system and endocrine system, and form a network each other, so these are role as a key factor in regulation of bone metabolism.
Cytokine which is intercellular signaling molecule is known as physiological active substance to regulate differentiation and function of each cell, and it is produced from various cells and regulates immune and hematopoiesis cells [9]. Most cytokines act through receptors on the cell surface of the target cells and its mechanism is similar to the action of the hormone, but it can be called site-specific because it acts topically to the level of cell. Some cytokines are generated from in the bone marrow cells, osteoblasts, macrophage, peripheral blood mononuclear cell (PBMC). It forms complex network between bone-related cells to regulate bone metabolism, so it is called bone metabolic cytokines [10,11]. Among them, interleukin (IL) -1 [12,13], IL-6 [14,15] and tumor necrosis factor (TNF)-α [16,17] are known to promote differentiation, proliferation, activation of osteoclast and strongly promote bone resorption, so they are reported as bone resorbing cytokines. Meanwhile, interferon (IFN) which is known as multifunctional cytokine to induce resistance against virus infection [18] is a glycoprotein isolated from lymphocytes and monocytes, and recent studies have shown that it affects bone formation and bone resorption directly or indirectly [19]. IFN-γ produced from human T cell is known that inhibits osteoclast formation [20,21] and inhibits osteoclasts differentiation [22]. I.e., it is suggested that IFN-γ acts on bone formation in contrast with bone resorbing cytokines such as IL-1, IL-6 and TNF-α. Therefore, precisely identifying bone metabolism by examining gene expression level of these osteogenic cytokines and bone resorbing cytokines is necessary to clarify the effects of exercise.
In the 1920s, pilates is designed by Joseph Hubertus Pilates (1880~1967), and it has been developed systematically combined with Yoga, Zen and the regimen of ancient Greece and Rome [23]. Pilates continuously repeat muscle exercise to strengthen the muscles without pain, it mainly train the mind and body organically through breathing exercise [24], and it is known that it contributes to stabilize center of body and erector spinae [25]. Pilates exercise divided into floor-based pilates which exercise mainly strength and flexibility on the mats with resistance band, ball and foam roller, and specialized equipment pilates which exercise using reformer, chair, barrel and so on. From the past, pilates is known as useful exercise for balance [26] and flexibility [27], but in the recent according to systematic review from Wells et al. [28] with total of 2,182 papers, it has been found that pilates exercise effects on improvement of center stability, strength, flexibility, muscle coordination, posture, breathing, etc. It is well known that exercise prevents osteoporotic fractures [29], and it is found in a recent study that pilates exercise is also good to improve osteoporosis which is a problem in the elderly people. Granacher et al. [30] said that pilates exercise prevents fractures by osteoporosis through improvement muscle strength and prevention falling in systematic literature review. Also, Küçükçakır, et al. [31] studied with 60 postmenopausal osteoporotic women divided into two groups, the control group and the pilates exercise group, and they did pilates exercise twice a week for one year, so they reported that pilates improve the quality of life for patients with osteoporosis. Thompson [32] also said that pilates increases BMD of osteoporotic patients, and it prevents falls and fractures by improving muscle strength and balance. According to the study examining effects of pilates exercise on BMD of working women [33], when they applied pilates exercises 90 minutes per day, twice a week for 12 weeks, BMD was improved. Although improvement effect in BMD of pilates exercise is gradually revealed, most study is about long-term effect of pilates exercise, and the effect of single bout pilates exercise is still not found. Also, there isn’t any study about what mechanism is activated by pilates exercise and how pilates improve BMD.
In this study, we load single bout pilates exercise to elderly osteopenia women to examine bone metabolism changes by pilates. That is, blood was collected before exercise, immediately after exercise and 60 minutes after the recovery, and Ca metabolic markers in the serum were examined. On the other hand, PBMC were isolated and cultured, then bone metabolic cytokine mRNA expressed from mononuclear cells was studied under molecular biology.
METHODS
Objects and procedure
Among the subjects who wish to voluntarily participate in the experiment, 30 women over 65 years old without regular exercise, medications or smoking and heavy alcohol intake for 6 months were selected through a questionnaire. 11 people of osteopenia subjects were selected through the pre-test by measuring the body composition and BMD (Table 1). We explained experimental details, damages for the experiment and freedom to stop, and finally selected experimental subjects in accordance with the regulation of experiment with the consent. This study is designed repeated measures in the same group. First, we divided into two groups, non-exercising control group and pilates exercise group, and repeated measurement was performed. Non-exercising control group (Con group) took a rest in the chair without any activity in the lab at 1 week before exercise, and pilates exercise group (pilates group) performed pilates after 1 week. To clarify the change rate by pilates, we set up the control group in this experiment by measuring change of variables.
Anaysis factors and methods
BMD measurement and osteopenia evaluation
30 women elderly over 65 years old examined body composition by electrical resistance method (Biospace, Inbody 4.0, Kuynggi-do, Korea) to select subject for this study, and BMD of whole body was measured using bone mineral density equipment (DPX-L, LUNAR, USA) of dual-energy X-ray absorptiometry (Dual energy X-ray apsorptionmetry ; DEXA). Osteopenia is determined under T-score suggested by the World Health Organization (WHO), it was classified as normal (T-Score: > -1 SD), osteopenia (T-Score: -1~-2.5 SD) and osteoporosis (T-Score: < -2.5 SD) [34]. As a result, 11 people who determined as osteopenia including 5 patients with osteoporosis were selected finally.
Method of Pilates Exercise
Single bout pilates exercise was conducted floor-based pilates for 70 minutes using a resistance band and foam roller (Table 2). For warm-up and cool-down exercise, breathing, spine articulation with foam roller, massage, mermaid, side stretch were conducted for 10 minutes, and hundred, roll-up, single leg circles, shoulder bridge, half swan, superman, side leg series, rotator cuff, triceps were conducted three times each for main exercise for about 50 minutes. Exercise intensity is measured by ratings of perceived exertion (RPE), it was evaluated an average 10-14 during main exercise. The important guidelines to pilates exercise is doing slowly, precisely and repeating several times while keeping the center of the body rather than doing many actions with speed, so we make them focusing the motion slowly.
Blood Sampling and Blood Analysis
Before the blood sampling, participants were suggested to maintain a regular eating pattern and to limit physical activity. Blood sampling was conducted after participants arriving the laboratory with empty stomach more than 12 hours, and blood in the rest (Baseline; B) was collected 30 minutes before start of exercise. And then plates exercise was performed for 70 minutes, 10 ml of blood was collected immediately after exercise (Post-Ex; P) and after 60 minutes (recovery; R) in each. At the non-exercise state, sampling was conducted without exercise at the same time (Fig. 1). Total RNA was extracted from PBMC which is separated from 4ml blood sample to examine mRNA expression in bone resorbing cytokine. The serum was separated from remaining 6ml blood sample centrifuged at 3,000 rpm for 15 minutes, and then the calcium metabolism markers and CK concentration were analyzed.
Cytokine mRNA Expression from PBMC
PBMC Isolation
EDTA treated on approximately 4ml of blood sample to PBMC extraction, the same volume of PBS (phosphate-buffered saline) was added to dilute. And layered 4 ml of Ficoll-Paque (Pharmacia, Sweden), then centrifuged 400 × g at room temperature for 20 min. The separated PBMC at the boundary between the blood and the Ficoll-Paque was obtained, then phosphate-buffered saline were added 2 times and washed to obtain pure PBMC.
Total RNA Extraction
PBMC isolation and total RNA extraction is conducted by modified method of Pacifici et al. [35], the entire process was operated in a sterile condition. Total RNA was extracted from separated mononuclear cells through modified acid guanidinium-thiocyanate-phenol-chloroform extraction method [36] using RNAzol Kit (Guadinethiocyanate 4M, 2-metrcap-toethanol 0.1M, Phenol; TEL TEST INC., USA). Briefly, 0.5ml of RNAzol were put in PBMC to dissolve cells, 0.05ml chloroform was added and mixed on ice for 5 minutes, then centrifuged 12,000 × G at 4 ℃ for 15 minutes. After centrifuge 0.5ml of isopropyl alcohol was added to the supernatant to extract total RNA, the extracted RNA was confirmed using a spectrophotometer. Finally extracted RNA was kept at -80℃ in ethanol until the measurement.
Complementary DNA (cDNA) Synthesis by Reverse Transcription
The cDNA synthesis was conducted using AccuPower RocketScript ™ Cycle RT PreMix (Bioneer. KR). Each RNA preserved with 75% alcohol (-80 ℃) dried at room temp-erature for 15 minutes, it dissolved with 10 μl of DNase/RNase free double distilled water. According to the manufacturer's instructions, premix including components necessary to cyclic reverse transcription (CTRT) such as reverse transcriptase, oligo (dT) 20, dNTPs, reaction buffer and primers were added in RNA lysate, and DW was added to make the total amount of 20 μl, then cDNA was synthesized. The annealing conditions were at 37°C for 30 seconds, cDNA synthesis did at 50°C for 4 minutes, the reverse transcription reaction to remove secondary structure of RNA template was conducted for 30 seconds at 55 °C and repeated 12 times. To confirm DNA extraction, DNA concentration was measured in eluent by A260/A280 ratio using spectrophotometer (ASP-2680, CellTA Gen, KR), then it was kept in -20 ℃.
Real-time PCR
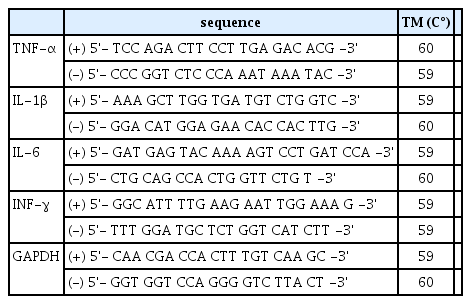
To analyze cytokine mRNA, we performed cDNA amplifying by Real-time quantitative polymerase chain reaction (Real-time PCR). Cytokines measured in this study were TNF-α, IL-1β, IL-6 and INF-γ, and analyzed relative amount with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA which is housekeeping gene. Forward primer and reverse primer which is react with synthesized cDNA in reverse transcription reaction were designed using Primer ExpressTM (Applied Biosystems, Foster, CA, USA) (Table 3). The following is PCR condition, 10 μl of Power SYBR Green PCR Master Mix (Applied Biosystems), 1 μl of 5pMol the forward primer and the reverse primer and 1 μl cDNA were added in each well, finally mixed with Diethylpyrocarbonate (DEPC) treated water, then we performed PCR with total amount of 20 μl solution. Pre-denaturation was done for 5 minutes at 95 ℃, and denaturation was conducted for 15 seconds at 95 ℃ each cycle, annealing step was done at 59-6 0℃ for 60 seconds and repeated 40 times, and then finally reaction at 65~95℃ for 1-5 seconds per each step was performed to melt curve. For SYBR Green analysis, C1000 Thermal Cycler (CFX96 real-time system, Bio-Rad, US) was used, the expression level of each target mRNA was corrected by the amount of GAPDH mRNA.
Serum Calcium Metabolic Marker and CK Concentration Analysis
The serum was isolated from 6ml of blood by centrifuging at 3,000 rpm, and it was stored in a freezer at -80℃. The concentrations of serum Ca, Alb, IP, Mg, ALP and CK was measured by Colorimetry method using Clinimate CA (SEKISUI Chemical Co. Ltd., Japan), ALB plus (Roche Diagnostics, Mannheim, Germany), Clinimate IP (SEKISUI Chemical Co. Ltd., Japan ), Roche Integra 800 electrode (Roche Diagnostics, Mannheim, Germany), Modular DDP (Roche Diagnostics, Mannheim, Germany) and Hitachi 7600-110 (Hitachi, Japan), respectively. To correct the Ca concentration which is undervalued by abnormal serum protein, Ca(mg/dl) - Alb(g/dl) + 4.0 formula was used [37]. CK was measured by IFCC method using AU 680 (Beckman coulter, USA).
Data Processing
The average, standard deviation, standard error of all data was calculated using SPSS for Windows (ver. 20.0) statistical program. 2-way repeated measure ANOVA method was performed for statistical analysis to analyze the difference (2 by 3) between the times (three times: before exercise, immediately after exercise and during recovery) and two kinds of strength (non-exercise and pilates exercise). All statistical significance level (α) is set at less than 5% (p < .05).
RESULTS
Comparison of serum Ca metabolic markers and CK after a single bout pilates exercise.
Serum Ca, adjusted Ca, P, Mg, Alb, ALP, CK concentration between the two groups were measured at the rest status 30 minutes before exercise (Baseline; B), immediately after 70 minutes of pilates exercise (Post-Ex; P) and 60 minutes after recovery (recovery; R), and the results are shown in <Table 4>. <Fig. 2> shows change rate of each variable based on pre-exercise rest status before the exercise. According to 2-way repeated measure ANOVA, serum Ca showed significant differences between the times, so it was significantly decreased at immediately after exercise and recovery (p < .05, p < .01). Adjusted Ca is also significantly decreased at immediately after exercise and recovery (p < .05, p < .01). Serum P appeared significant interaction between group and time (p < .001), so the control group showed a tendency to increase but the pilates exercising group significantly decreased 91% after exercise and 87% during recovery (p < .05) (Fig. 2(C)). Serum Mg indicated significant differences between the times, it was increased significantly immediately after exercise and during recovery (p < .05, p < .01), while ALP was significantly decreased during recovery (p < .01). Serum CK appeared significant interactive effect between group and time (p < .05), whereas no significant change in the control group, the pilates exercise group was significantly increased immediately after exercise and during recovery about 127% and 137%, respectively (p < .05) (Fig. 2(F)).

The change rate (%) of serum Ca metabolism markers and CK at before and after a single bout pilates exercise. (A), Change rate of Serum Ca; (B), Change rate of Adjusted Ca; (C), Change rate of Serum P; (D), Change rate of Serum Mg; (E), Change rate of Serum ALP; (F), Change rate of Serum CK. Ca, calcium; P, phosphate; Mg, magnesium; ALP, alkaline phosphatase; CK, creatine kinase. B, baseline; P, immediately post exercise; R, recovery of 60 minutes after pilates exercise. †p < .05, ††p < .01 significantly different ingroup × time interaction; * p < .05, *#x0002A; p < .01 significantly different from baseline exercise; # p < .05, ## p < .01 significantly different from post pilates exercise.
Comparison of expression in TNF-α, IL-1β, IL-6 and INF-γ mRNA from PBMC after a single bout pilates exercise.
<Table 5> indicates mRNA expression changes of TNF-α, IL-1β, IL-6 and INF-γ in PBMC between two groups at 30 minutes before the exercise, at immediately after the exercise and at 60 minutes after recovery. <Fig. 3> shows change rate of each variable on the basis of the rest status before the exercise. According to 2-way repeated measure ANOVA, TNF-α mRNA expression showed significant differences between groups (p < .01), the control group tended to decrease, whereas the pilates exercise group tended to increase significantly at immediately after exercise and 60 minutes after recovery (Fig. 3(A)). IL-6 mRNA expression was also shown significant differences between groups (p < .05), the control groups tended to decrease, whereas the pilates exercise group showed a significantly increased tendency (Fig. 3(C)). IL-1 and INF-γ mRNA expression was increased in the pilates exercise group like as TNF-α and IL-6 mRNA expression, but there was no significant difference statistically.
The change of expression in TNF-α, IL-1β, IL-6 and INF-γ mRNA from PBMC after a single bout pilates exercise
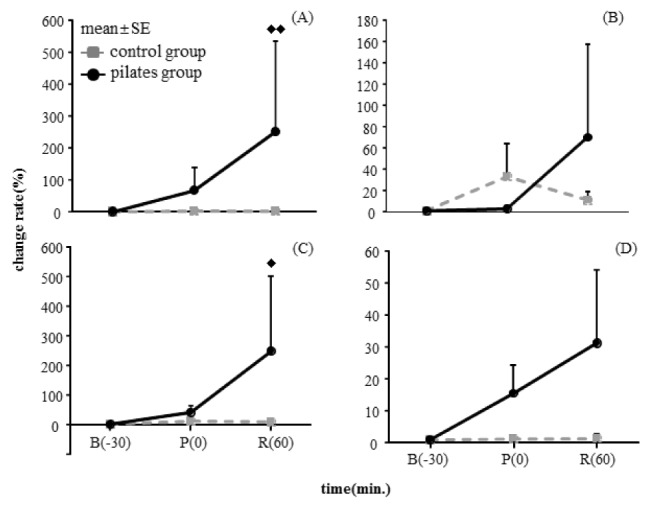
The change of expression of cytokine mRNA from PBMC at before and after a single bout pilates exercise. (A), Change of TNF-α mRNA expression; (B), Change of IL-1β mRNA expression; (C), Change of IL-6 mRNA expression; (D), Change of INF-γ mRNA expression. B, baseline; P, immediately post exercise; R, recovery of 60 minutes after pilates exercise. ♦ p < .05, ♦♦ p < .01 significantly different between groups.
DISCUSSION
To consider impact of single bout floor-based pilates exercise on bone metabolism, elderly osteopenia women who bone resorption is progressing are targeted in this study. They divided into non-exercise group and exercise group, and loaded pilates exercise. Blood was taken at before and immediately after exercise and 60 minutes after recovery, then calcium metabolism markers and mRNA expression of bone metabolic cytokines from PBMC was examined. As a result, the serum Ca and adjusted Ca decreased significantly after exercise and during recovery (p < .05, p < .01) and serum Mg was significantly increased (p < .05, p < .01), but there was no significant difference with control group. Mineral concentrations in blood were changed by diurnal rhythm so it was confirmed that the pilates exercise tend to make larger the fluctuation. However, serum P increased in the control group, whereas it was significantly reduced more after exercise and during recovery in the pilates exercise group (p < .001). So it was suggested that temporary hypophosphatemia occur possibly by pilates exercise. Prolonged hypophosphatemia can cause rickets [38], and it finally occurs osteomalacia even in normal serum Ca condition [39]. In addition, according to the report from Mehrotra et al. [40], when low calcium formula treated to rabbits for 10 weeks, hypocalcemia occurs accompanied by hypophosphatemia, and ICTP (urinary C-terminal telopeptide of class I collagen) which is one of the bone resorption marker is also increased, so eventually bone mass loss is promoted. This study showed bio adaptation result to long-term exercise for 10 weeks, it may be different with temporary body reaction by single bout exercise. For example, like as temporary exercise increases heart rate but long-term exercise reduces the resting heart rate, temporary reaction and long-term adaptation may show different result. In the recent study about effects of treadmill exercise for 16 weeks on bone metabolism in hypertensive rat [41], serum P didn’t change in the exercise group and the non-exercise group. In the research about effects of resistance exercise for 12 weeks on bone mineral density in rat [42], it is reported that significant difference could not be found in the exercise and the non-exercise group, so it means single bout exercise may increase serum P level but serum P is likely to be maintained by constancy of P when the exercise lasts long time.
In the results of this study, while serum CK does not change in the control group, it was confirmed that significantly increased just after pilates exercise and during recovery (p < .05). CK is an enzyme that converts adenosinedi-phosphate (ADP) and creatine phosphate to adenosine tri-phosphate (ATP) and creatine, and it presents large amount in muscle [43]. In general, it is altered significantly by exercise and it is highly correlated with exercise intensity, time and the amount of training. Also, it is used as biochemical markers of muscle damage [44], and it has been reported that single bout exercise increases the CK level [45]. Gleeson et al. [46] also reported that CK level increased significantly with muscle pain when bench stepping exercise was conducted for 40 minutes targeting the average 20-year-old male and female students. Resistance exercise using elastic resistance bands and foam roller was performed with pilates exercise in this study, and in other words the exercise was resistance movement enough to cause muscle damage. The rating of perceived exertion (RPE) of pilates exercise conducted in this study is around 10 to 14. When calculate % maximum heart rate using the average value of 68 years old, it can be regarded as significantly higher exercise intensity. Pilates can improve the balance of the muscle mainly using small muscles of the body [24], it strengthen core muscle which is involved in the stability of the abdominal and spine muscle [25], and pilates also includes movement to promote the flexibility. American College of Sports Medicine (ACSM) recommends that it consume about 200~400 kcal per single bout exercise for health promotion [47,48], and 60~80% VO2max is recommended for appropriate exercise intensity [49]. However, most of these are intensity guidelines for aerobic exercise, so it is very different from the exercises to emphasize resistance and flexibility such as pilates. Thus, even the exercise intensity of pilates is very difficult to define, this study revealed that pilates has sufficient effect as resistance training exercise through increasing CK level significantly.
Meanwhile, we reviewed TNF-α, IL-1β, IL-6 and INF-γ mRNA expression changes in PBMC between the two groups at 30 minutes before the starting exercise, at immediately after pilates exercise and 60 minutes after recovery. In the result, it was shown that TNF-α and IL-6, bone resorbing cytokine, mRNA expression was significantly increased in the pilates group (p < .01, p < .05). This is the most important finding in this study. It means that overexpression of bone resorbing cytokine is promoted by single bout pilates exercise. IL-6 and TNF-α is generated from bone marrow cells, macrophage and PBMC, and particularly they promotes activation and proliferation of osteoclasts, also bone resorption is promoted by bone absorbing cytokines [12-14,17]. Ishimi et al. [50] reported that IL-6 and IL-1 cooperation and promote bone resorption in the experiment with bone of newborn mice, and Suda et al. [51] uncovered as IL-6 and TNF-α facilitate the formation of osteoclasts proportionately. Like these, it is known that bone metabolism is regulated by cooperation with bone resorbing cytokines. In immobile bone atrophy occurred by long-term bed rest, it is also reported that these bone resorbing cytokine regulate bone metabolism [52], and it is revealed that cytokine mRNA expression was increased in osseous tissue from hind limb suspension rat [53].
In the recent study, when single bout high-intensity anaerobic exercise using the bicycle ergometer was performed, IL-6 and TNF-α, bone resorbing cytokines, mRNA was significantly increased with increasing lactic acid at 15 minutes after exercise, and acidification of the blood by high-intensity exercise increased the expression of bone resorbing [54]. However, the recent research about single bout anaerobic exercise below the threshold showed that TNF-α and IL-6 mRNA expression is not increased [55]. These results suggest that TNF-α and IL-6 expression level is increased in response to exercise at least exercise intensity harder than the threshold, and they have possibility to promote bone resorption in the cell level. According to the result which is approximately 1.4-fold increasing in CK and TNF-α and IL-6 mRNA expression, single bout pilates exercise conducted in this study is thought to be enough to stimulate the bone metabolism. In addition, the inflammatory response according to skeletal muscle injury could be a direct cause of increasing TNF-α and IL-6 mRNA expression level. But DeRijk et al. [56] reported that it also take place by adrenaline, and it is associated with the exercise intensity as shown in the study of Ostrowski et al. [57] which says plasma IL-6 is proportional to lactate. Meanwhile, it is known that IFN-γ mainly produced from T cell suppresses the formation of osteoclasts [20] and inhibits the differentiation of osteoclasts [22], so IFN-γ is known to be related with bone formation in contrast to TNF-α and IL-6. In this study, IFN-γ mRNA expression showed tendency to increase without statistically significant difference, which is said that single bout pilates exercise promotes bone formation temporarily. In other words, it could be high turnover bone metabolic state which is bone resorption and bone formation are promoted. Meanwhile, changes in cytokines by long-term exercise may be different from singe bout exercise. Marques et al. [58] performed combined exercise for 32 weeks which performed resistance training twice a week and aerobic exercise once a week, and examined BMD and concentrations of cytokines in serum. They showed muscle strength increased with BMD, but TNF-α showed no change and IL-6 showed a decreasing tendency. That is, it is possible to be increased temporarily after single bout exercise, but the expression amount is likely to be changed when exercise is prolonged. On the other hand, changes in INF-γ according to long-term exercise appeared differently with IL-6 and TNF-α, so there are reports that INF-γ decreased eight weeks after combined exercise [59] and INF-γ didn’t change after exercise for 8 weeks [60].
Consequently, as a result of single bout pilates to elderly osteopenia women, CK increased and P reduced, and TNF-α and IL-6, the bone resorbing cytokine, mRNA expression was increased as well as INF-γ mRNA expression level was increased. These results suggested that muscle damage and temporary hypophosphatemia is occurred after single bout pilates exercise, and it leading high turnover bone metabolic state with to activate both of bone formation and bone resorption.
Acknowledgements
This article was supported by the 2013 Dongduk Women's University grant.