Serum Vitamin D status and its relations to body fatness and fitness and risk factors in young adults
Article information
Abstract
The study examined the relations of serum vitamin D levels to body fatness, cardiorespiratory fitness (CRF), and metabolic risk factors in young adults in Korea. A total of 593 young men completed a health examination, body fatness, maximal treadmill exercise test, and assessment of metabolic risk factors. Participants were classified by serum vitamin D levels as deficient (< 20 ng/mL), insufficient (20~30 ng/mL), and sufficient (> 30 ng/mL). Body fatness, CRF, and metabolic risk factors were evaluated according to serum vitamin D classification. Significant inverse trends in body fatness and metabolic risk factors were observed, as was a significant linear trend for CRF across incremental vitamin D categories in this study population. Serum vitamin D levels were negatively associated with body fatness parameters, blood pressures, total cholesterol, triglycerides, and insulin and positively associated with high-density lipoprotein cholesterol and CRF. Compared to the BMI-based lean group, the obese groups had significantly higher odds ratio for serum vitamin D insufficiency before and after adjusting for age, CRF, and physical activity. Similarly, compared to percent body fat- and waist circumference-based lean groups, the obese groups had significant higher odds ratios for serum vitamin D insufficiency. In conclusion, the current findings of the study suggest that along with vitamin D intakes, body fat loss and outdoor physical activity should be promoted as non-pharmacologic means to improve metabolic risk factors in young adults.
INTRODUCTION
Recent prior studies report that the deficiencies in lowering the level of the serum 25(OH)D have graudally increased and the vitamin D deficiency may play a role as a direct risk factor which induces various metabolic diseases including obesity, diabetes, metabolic syndrome and cardiovascular diseases [1]. In particular, the hypothesis that the global increase in the obesity prevalence rate is the major cause of the vitamin D deficiency with dynamic increase. In the related prior studies, Looker et al. [2] shows that the obesity is negatively correlated with the 25(OH)D level and Wortsman et al. [3] compare the healthy and obese women with more than 30kg/m2 of the BMI and the women with normal weight in the same age group and report that the obese women contain significantly low serum vitamin D but significantly high parathyroid hormone compared to the normal women.
In this respect, the clear causes of the vitamin D deficiency among obese patients have not been clearly found out. However, the causes found so far are: First is the inhibition of the vitamin D activation in the skin tissues due to insufficient exposure to the UV by the behaviors including the limited mobility or the avoidance of outdoor activity [4]. Second is due to the excessive deposition of the vitamin D with the feature of fat soluble on the body fat. Even worse, the blood vitamin D is not only related to the obesity but also is known to prevent the insulin resistance and diabetes through the insulin irritability.
The vitamin D receptor (VDR) is expressed in the whole body and known to stimulate the insulin excretion, insulin receptor expression and insulin reaction [6]. Chiu et al. [7] perform the data analysis be measuring the stages 1 and 2 of the insulin excretion from the pancreas and the insulin sensitivity using a hyperglycemic clamp method for 126 healthy Californians and report that the serum 25(OH)D level shows the positive correlation with the insulin sensitivity and negative correlation with the stages 1 and 2 of the insulin excretion. The most important fact in the study is that the patient with deficient blood vitamin D (20 ng/mL) shows relatively high risks of the insulin resistance and the metabolic syndrome. The data from the Third National Health and Nutrition Examination Survey (NHANES III) between 1988 and 1994 showed that blood vitamin D is an independent predictor of metabolic syndrome [8]. Fung et al. [9] conducted a follow-up survey on the attack rate of the metabolic syndrome by the vitamin D intake and administration from the food for 4727 young Cocasian and black people who join the Coronary Artery Risk Development in Young Adults Study for 20 years and reported that vitamin D intake played an independent role in lowering the metabolic syndrome. The previous studies suggest that blood vitamin D level is closely related to various metabolic diseases including metabolic syndrome like obesity, hyperglycemia and insulin resistance.
Such prior epidemiological research results estimate that insufficiency and deficit in vitamin D may contribute to the increased risk of being exposed to various types of metabolic diseases like obesity and diabetes. It is known that activation of vitamin D inhibits the preadipocytes cell differentiation in the fat cells. Sato & Hiragun [10] firstly report that the activated vitamin D injection inhibits the preadipocytes cell differentiation through the cell culture test and the result is proved by other tests using different kinds of cell lines [11, 12]. Also, Zhuang et al. [13] report that the vitamin D3 injection inhibit the cell differentiation and proliferation of the porcine preadipocytes cells and the gene expression of the cell differentiation marks like PPARγ, retinoid X receptor α. Then, Maestro et al. [14] report that the vitamin D3 injection increases the insulin receptor expression in the U-937 human monocytic cells.
The biological mechanism where the vitamin D is engaged in the diabetic prevention may be found from the fact that the vitamin D contributes to the calcium homeostasis. Christakos et al. [15] report from the recent experiment that the calbindin, a calcium combination protein, inhibits the cytokine which inhibits the insuline secretion from the pancreas beta cells. Bourlong et al. [16] and Ayesha et al. [17] report that the vitamin D deficiency decreases the switch from the pro-insulin to the insulin. In addition, it is known that the vitamin D inhibits the cytokine expression related to edema like the IL-1, IL-6, IL-8 and TNF-α [18].
The serum vitamin D level is reported to have significant correlation with the heart-lung physical fitness from foreign prior studies. For example, the data produced by the Cooper Center Longitudinal Study report that the heart-lung physical fitness shows positive correlation with the serum vitamin D level of male [19] and female [20] and the chronic kindey patients show positive correlation between the heart-lung physical fitness and the blood vitamin D level [21]. Similarly, Ardestani et al. [22] report that the serum vitamin D level shows significant quantitative correlation with the maximum oxygen intake directly measured by the exercise test from the survey for 200 healthy male and female adults. Reports that the heart-lung physical fitness plays a role in the risk factor of various metabolic diseases as an independent prediction factor and is closely related to the serum vitamin D level have been released from foreign prior studies with consistency but the clear biological mechanism has not been specifically explained yet.
Academic interest in the vitamin D has increased in that the blood vitamin D is related to the metabolic risk factor based on the national nutrition survey of Korea [23] and it is reported that the blood vitamin D deficiency among male shows positive correlation with the body fat and negative correlation with the total fat but no consistency exists in the female [24]. However, the prior survey by the research team finds out that the local piror studies limit the factors in affecting the blood vitamin D level in the physical composition factor including obesity and there has been no case in verifying the correlation with the physical activities or heart-lung physical fitness. Foreign prior studies report that not only the physical composition factors, but also physical activities and heart-lung physical fitness are the lifestyle factors which affect the blood vitamin D level and it is desirable to comprehensively contain such factors. Also, the national nutrition survey states that the blood vitamin D deficiency is more severe in the 20s rather than the established [25] but the studies on the age group are rare. Based on the situation, the study focuses on comparing the correlation between the blood vitamin D level, obesity index, heart-lung physical fitness and risk factors in the metabolic diseases for male university students with no sever disease diagnosed by the medical staff and health.
METHODS
Subject
The subjects of the study are male students in the university of S in Jangan-gu, Suwon, Gyeonggi (N = 593) whose appearing health is fine and voluntarily join. The researchers sufficiently explain the contents and the purpose of the study to the subjects and let the subjects sign the prior agreement on the experiment. All the measurements are performed in the exercise physiology lab at the university of S with stomachs empty for 10-12 hours.
Measurement and analysis item
Body composition factor: The body composition factors include the body mass index (BMI), precent body fat (%BF) and the waist-to-hip ratio (WHR). The height (cm) and weight (kg) are measured by the automatic measurement (DS-102, Jenix, Korea), calculated with BMI = Weight (kg) / Height (m)2, the obesity is measured the benchmark of BMI 25 kg/m2 (IDF, 2005) and the %BF is measured by the X-Scan Body Composition Analyzer (JAWON Medical Co., Seoul, Korea). The waist circumference is measured with the tape measurement in the horizon with the floor, passing the belly button in parallel while the subject is standing erect.
Metabolic risk factor: The blood pressure in the stabilized state is measured twice by the automatic blood pressure gauge after being stabilized for more than 10 minutes and their average value is taken. The blood factors include the neutral fat, total cholesterol, high density lipid protein cholesterol, blood level at empty stomach and insulin and the blood sugar level and insulin are calculated with the homeostatsis model assessment for insulin resistance (HOMA-IR). The venous blood is taken from the brachial veins after 12 hours of empty stomach to meausre the blood lipid and blood sugar level, the plasma is separated from the taken blood by the centrifugation (3000 rpm, 15 minutes), the neutral fat, total cholesterol, high density lipid protein cholesterol and blood sugar level are analyzed by the Vitro Chemistry DT60 II (Johnson & Johnson, NY, USA) and the insulin is analyzed by the Human Insulin ELISA kit (ALPCO Diagnostics). The HOMA-IR is calculated by the equation of [Insulin (μIU/ml) × blood sugar level (mmol/L)/22.5] proposed by Matthews et al. [26].
The blood vitamin D level: The blood vitamin D level is measured by the LIAISON 25(OH) Vitamin D TOTAL Assay with the measurement fo the DiaSorin LIAISON automated analyzer (Italy, DiaSorin S.P.A). The blood vitamin D level is classified as deficiency (< 20 ng/ml), insufficiency (> 20 - < 30 ng/ml) and sufficiency (> 30 ng/ml) proposed by Holick et al. [27].
Maximum oxygen intake per minute: The maximum oxygen intake is measured by the treadmill (Medtrack ST 65, Quinton, USA) and the gas analyzer (True-one, Quinton, USA). The exercise test protocol performs the gradual maximum exercise test using the Bruce protocol. The benchmark for reaching the maximum capacity is with more than 1.15 of the respiratory exchange ratio (RER), more than 17 of the RPE and without VO2 increase despite increasing the exercise strength.
Lifestyle factors: The self-produced questionnaire based on the Physical Activity Readiness Questionnaire (PAR-Q) and a health history one [28] were used to collect data regarding the frequency of moderate and vigorous physical activities and smoking rate, respectively.
Data analysis
All the data measured from the study are with the value of average ± standard deviation and the statistical methods used in the study are as follows. A log 10 transformation was used for any variable without standard distribution. First, the Kruskal-Wallis test was used to analyze the linear trends in obesity, cardiorespiratory fitness levels, and metabolic risk factors across the serum vitamin D level (deficiency, insufficiency, sufficiency). Second, Pearson correlation was used to calculate the serum vitamin D level and the measurement factors. Third, logistic regression analyses were used to calculate the odds ratio of being exposed to blood vitamin D deficiency between non-obese and obese groups. Data analyses were conducted with the SPSS-PC (version 18.0) program and the selection or rejection of the configured research hypothesis is performed at the credibility level of p = 0.05.
RESULTS
Compare obesity index, heart-lung physical fitness and risk factor for groups based on the vitamin D level
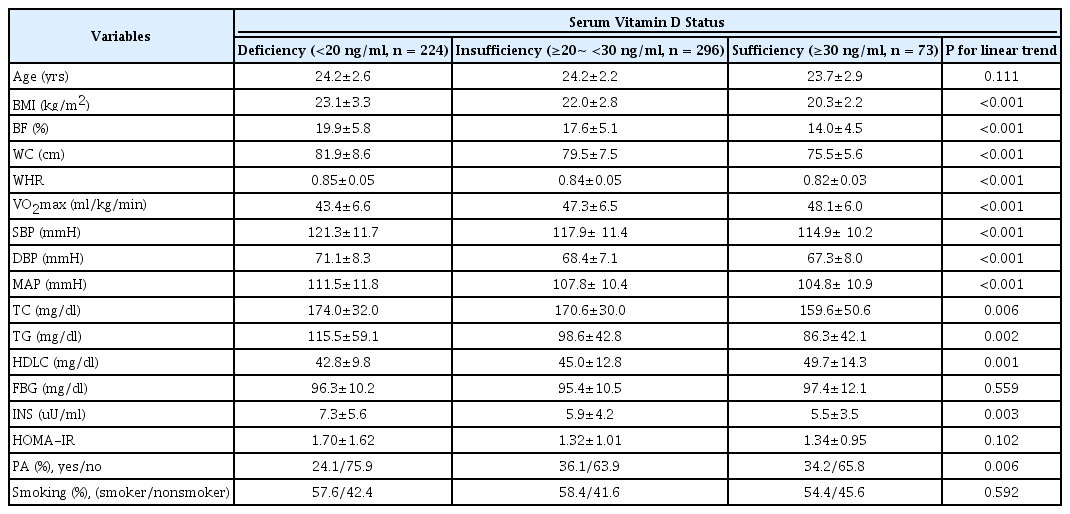
[Table 1] compares the average difference between the obesity and the physical fitness index by categorizing the deficient, insufficient and sufficient groups based on the serum vitamin D level measured at the empty stomach from 593 voluntary male university students who attended the physical class at the university of S from 2008 to 2009. The result shows that as the serum vitamin D level increases from the deficiency to insufficiency and to sufficiency, the average values of obesity indexes including the BMI, body fat and waist circumference significantly decrease but the average of the heart-lung physical fitness significantly increases (Table 1).
Like the obesity and the physical fitness index, the result of comparing the average value of the metabolic risk factors for groups based on the serum vitamin D shows that the systolic blood pressure, diastolic blood pressure, total cholesterol, neutral fat and insulin proportionally decrease but the high density lipid protein cholesterol significantly increases. It shows through the questionnaire that the vitamin D level is qualitatively proportional to the physical activities higher than the weekly severity but no significant difference in the smoking rate is found among the groups.
Additionally, the Pearson correlation analysis shows that the serum vitamin D level contains the negative correlations with the BMI (r = -0.292), body mass ratio (r = -0.325), raist circumference (r = -0.260), systolic blood pressure (r = -0.179), diastolic blood pressure (r = -0.176), total cholesterol (r = -.146), neutral fat (r = -0.251) and insulin (r = -0.204) but positive correlation with the maximum oxygen intake per minute (r = 0.326) and high density lipid protein cholesterol (r = 0.215) (Table 2).
Relative risk of the exposure to the vitamin D insufficiency and deficiency
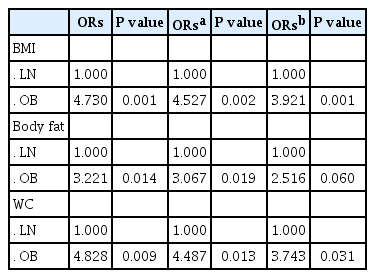
[Table 3] shows the relative risk which may be exposed to the serum vitamin D deficiency due to increasing the obesity indexes including the BMI, body fat and waist circumference by the logistic regression analysis. It is found out that the relative risk of the obesity group exposed to the vitamin D deficiency and insufficiency is 3.9 times higher than the normal group if the age is adjusted (p = 0.002) and still higher 3.7 times if the individual differences in the heart-lung physical fitness and physical activities are additionally adjusted (p = 0.001) for the BMI.
The relative risk in the obesity group which may be exposed to the vitamin D deficiency and insufficiency is about 3.0 times significantly higher than the normal group based on the body fat ration under the age adjustment (p = 0.019) but it shows that the relative risk is about 2.5 times (p = 0.060), not significant but showing tendency under the additional adjustment of the physical fitness and physical activities in the individual difference.
Finally, the relative risk which may be exposed to the vitamin D deficiency and insufficiency due to increasing an indicator in the abdomen obesity, the waist circumference is significantly higher about 4.5 times (p = 0.013) under the age adjustment and 3.7 times higher despite the adjustment of the individual difference in the heart-lung physical fitness and physical activities (p = 0.031).
DISCUSSION
The study categorizes the healthy male university students deficient, insufficient and sufficient based on the serum vitamin D level, compares and analyzes the obesity index, heart-lung physical fitness and metabolic risk factors among the groups. The result shows that the average values of the BMI, body fat, waist circumference, blood pressure, total cholesterol, neutral fat and insulin significantly decrease as the serum vitamin D level improves from deficiency to insufficiency and sufficiency and significantly increase in proportion to the heart-lung physical fitness and high density lipid protein cholesterol. The result is verified again by the Pearson correlation analysis and specifically, it is found out that the vitamin D level shows significantly negative correlation with the obesity indexes and metabolic risk factors including the BMI, body fat and waist circumference and significantly positive correlation wih the heart-lung physical fitness and high density lipid protein cholesterol. The impact of the age, obesity index, heart-lung physical fitness and physical activity level adjusted by the correlation anlalysis using the multiple linear regression analysis for the factors with significant correlation with the serum vitamin D level shows that the impact of the serum vitamin D level on the total cholesterol and high density lipid protein cholesterol are affected to some extent by the lifestyle factors including the obesity index, heart-lung physical fitness and physical activity level but the impact of the serum vitamin D level on the blood pressure (average arterial pressure) and insulin level are independent from the age, obesity index, heart-lung physical fitness and physical activities. Finally, the calculation of the relative risk which may be exposed to the serum vitamin D level deficiency due to the obesity based on the BMI, body fat and waist circumference shows that the relative risk which may be exposed to the serum vitamin D level deficiency due to the obesity is significantly higher than the normal weight despite the statistical adjustment of the impact from the heart-lung physical fitness and physical activities.
The results of the study mentioned above are judged consistent with some prior studies. For example, Ford et al. [8] survey the correlation between the serum vitamin D level and the metabolic syndrome based on the data for the American national nutrition survey and report that the group with the metabolic syndrome shows significantly low serum vitamin D level compared to the normal group and the serum vitamin D level shows significantly negative correlation with the abdomen body fat, hyperlipidemia and hyperglycemia. Dong et al. [29] state that the serum vitamin D level has significantly positive correlation with the physical activities and heart-lung physical fitness in a horizontal study on the African Americans, cocasian children and youth. Valtueña et al. [30] investigated whether body composition and physical fitness is associated with blood vitamin D levels in 1006 European youths aged between 12.5 and 17.5 years old who joined The Healthy Lifestyle in Europe by Nutrition in Adolescence-CSS study. The result shows that the heart-lung physical fitness and the BMI have significantly independent correlation with the blood vitamin D level for male participants but the grip has significantly independent correlation with the blood vitamin D for the female participants. Ardestani et al. [22] report that the serum vitamin D level shows significantly quantitative correlation with the maximum oxygen intake per minute in the survey for 200 adults. The Cooper Center Longitudinal Study showed that serum vitamin D levels for male and female adults were associated with the heart-lung physical fitness and the interesting observation was that the calculation of the relative risk showed that the blood vitamin D level was significantly higher in the fit (excellent physical fitness) group compared to the unfit (low physical fitness) group even for all the groups classified based on the obesity index. Therefore, the results indicate that blood vitamin D level may be determined by the interaction between the body composition factors including the body fat and the heart-lung physical fitness.
Petchy et al. [21] confirmed that how the correlation between the blood vitamin D level and cardiovascular risk factors is affected by the muscular strength and cardiovascular endurance for the patients sufferring from the chronic renal insufficiency and showed that blood vitamin D level was inversely associated with the cardiovascular endurance and positively with the muscular strength. The quantitative correlation between the heart-lung physical fitness and the serum vitamin D level are consistently reported for the children, adolescents, adults, patients and senior groups. For example, Houston et al. [31] report the correlation result of the vitamin D level and physical fitness for the elderly people who participate in the InCHIANTI study. On the local basis, Kim et al. [24] analyze the data for the 4th national health and nutrition survey for 559 adults older than 19 years old and report that the blood vitamin D deficiency is related to metabolic risk factors including the obesity index, hyperglycemia, hyperlipidemia. Also, Chio et al. [25] report that the blood vitamin D deficiency is higher for the young generations of 10s and 20s and Park & Lee [23] report that the blood vitamin D level is closely related to the relative risk which may be exposed to the cardiovascular diseases for the senior order than 50 years old from the data for the national health and nutrition survey.
The originality of the study against local and foreign prior studies is that the study is performed on the healthy male subjects in their 20s and shows that the body composition and heart-lung physical fitness may become important lifestyle factors in determining the blood vitamin level for male adults in their 20s and the blood vitamin D deficiency and insufficiency may play a role as a risk factors which may cause the grouping of the risk factors in the various diseases or the metabolic syndrome. Even though the study may not clearly propose the causation for the fundamental result, it is guessed that according to the prior studies, degrading the heart-lung physical fitness due to insufficient physical activities, deficient exposure to the UV due to insufficient outdoor physical activities and inhibition of the vitamin D activation due to excessive body fat deposition [5] play a role as the major factor in the blood vitamin D deficiency and insufficiency among male subjects in their 20s.
The results of the study show that the serum vitamin D level is negatively correlated with the obesity index, blood pressure, blood lipid and insulin resistance index and positively correlated to the heart-lung physical fitness. Therefore, the serum vitamin D level may significantly decrease by the obesity due to excessive calorie intake and degrading heart-lung physical fitness by insufficient exercise and it is indicated that the following blood vitamin D insufficiency may directly or indirectly cause the metabolic syndrome including the hypertension, hyperlipidemia and insulin resistance. In addition, considering the fact that the deficient and insufficient blood vitamin D level is closely related to degrading the physical fitness due to excessive body fat accumulation and insufficient exercise, improving the physical fitness through outdoor activities which may supplement the vitamin and in particular, sufficient UV exposure ultimately minimize the blood vitamin D level deficiency and insufficiency and shall be a component in the required healthy lifestyle or intermediary program to prevent the risk of being exposed to the metabolic syndrome. Also, the logistic regression analysis shows that the obesity index is an independent risk factor which contributes to the vitamin D, indicating that it should be a key component of an intervention program to prevent the vitamin D insufficiency or the dietary control to reduce the body fat, as well as physical activities to improve the physical fitness.
Lastly, considering the fact the study is a horizontal study for the purpose of verifying the correlation among the measured factors, the causation for the result may not be certain. Therefore, it is judged that there shall be additional verification on the impact of improving the heart-lung physical fitness on the serum vitamin D level through exercise intermediary program, difference bewteen outdoor and indoor exercise intermediary programs which cause significant difference in the UV exposure time and the complex treatment with the serum vitamin D supplement and exercise. Also, future studies should consider nutritional supplements and dietary intake of vitamin D intake.
Acknowledgements
The study is funded by the special research project in Dongseo University in 2013.