INTRODUCTION
Exercise-induced muscle damage is categorized into two types, namely acute muscle soreness and delayed onset muscle soreness (DOMS). DOMS occurs 8-10 hours after performing an exercise, with symptoms of pain appearing at 24-28 h. These symptoms alleviate 5-7 days later and are lost 8-10 days later [1,2]. Exercise-induced DOMS diminishes athletic performance by reducing the range of motion and maximum muscle strength and might lead to musculoskeletal and neurological disorders [3,4]. DOMS is assessed based on indicators, such as lactate, ammonia, myoglobin, lactate dehydrogenase (LDH), creatine kinase (CK), and aspartate aminotransferase (AST). CK is a typical enzyme used to determine musculoskeletal injury, where the CK levels continuously rise after a vigorous exercise. LDH is an oxidation-reduction enzyme that proportionately increases with exercise intensity. Furthermore, it increases more than twice after a prolonged endurance exercise [5]. AST and alanine aminotransferase (ALT) are particularly used as indicators of hepatocellular damage after a prolonged vigorous exercise. While ALT is present in the liver cells, AST is found in the heart, liver, and skeletal muscles. AST leaks from the muscles to the blood upon a muscle injury. An elevated AST concentration is an indicator of muscle injury [6].
Therapeutic interventions, such as stretching, massage, vibration, ultrasound, hyperthermia, cold therapy, acupuncture, microcurrent electrical therapy, and nutrition therapy have been introduced to alleviate DOMS symptoms [7-10]. Moreover, effects of branched chain amino acids (BCAA) in muscle tissue regeneration and restoration as a nutrition therapy have particularly gained attention in recent years. BCAA is the primary essential amino acid component of muscle proteins and comprises a group of three amino acids, including leucine, isoleucine, and valine that are oxidized in the skeletal muscles [11]. BCAA stimulates muscle tissue recovery and regeneration. Studies have reported on a decrease in exerciseinduced muscle injury by BCAA consumption. BCAA can increase the endurance capacity during an exercise by delaying muscle fatigue and reduces muscle pain [12-14]. Few studies have investigated changes in the muscle strength and the indicators of muscle injury [19-21] with BCAA administration [15-18]. However, there are limited studies on the maximum muscle strength and the indicators of DOMS-induced muscle damage to examine the effects of BCAA. Thus, this study aimed to analyze the maximum muscle strength and indicators of muscle damage to examine the effects of BCAA on DOMS. In other words, we first induced DOMS using an isokinetic exercise and analyzed the changes of knee peak torques and blood AST, CK, and LDH after BCAA administration.
METHODS
Participants
Twelve men with majors in physical education from C University were enrolled. They were assigned to the BCAA and placebo group in a double-blinded method. We recorded repeated measurements with a 6-week interval between the groups. The participants were healthy individuals without any clinical disease. All participants provided their signed informed consent. The mean age was 23±1.4 years, with a mean height of 175.7±3.2 cm and a mean body weight of 73.2±5.2 kg.
Experimental procedures
Experimental design is shown in Figure 1. We measured the knee extension peak torque and flexion peak torque, before and after BCAA administration, before and after inducing DOMS. More specifically, the first isokinetic exercise measurement was recorded (D0) after administering BCAA for 5 days before an exercise (-D5, -4D, -3D, -2D, and -1D). In contrast, we recorded the second isokinetic exercise measurements (D3) after administering BCAA for 3 days (D0, D1, and D2) following an exercise to induce DOMS. Furthermore, we obtained blood samples before (pre-D0), immediately after (post-D0), and 24 h (D1), 48 h (D2), and 72 h (D3) after the exercise. This helped us analyze changes in the indicators of muscle injury based on BCAA administration, before and after inducing DOMS.
Exercise-induced DOMS
DOMS was induced by performing an isokinetic exercise using an isokinetic testing system (Cybex CSMi Humac NORM, 770), based on the methods proposed by Cornish & Johnson [22]. In other words, the participants performed six sets (10 repetitions per set) of isokinetic exercise at 60% of the knee extension peak torque at 120˚/sec angular velocity. They were provided a break for 10 s between the sets. We recorded the measurements between 9-11:00 am at the pretreatment baseline (D0), following 5 days of BCAA administration, and 72 h after the exercise (D3). We measured the knee extension peak torque and flexion peak torque.
BCAA supplement
BCAA supplements were administered twice daily from 5 days before the exercise to induce DOMS (-D5) and for 72 h after the exercise (3D), for a total of 8 days. AMINOVITAL 400 (A, Japan) was used as the supplement. They were consumed before breakfast and lunch. Each pouch (total calorie 18.7 Kcal) contains 3 g, 4.0 g, and 1 mg of BCAA, protein (leucine 1.60 g, isoleucine 0.43 g, valine 0.44 g, and other amino acids 1.51 g), and vitamin B, respectively. Considering the maximum recommended dose of 1.6 g/kg BCAA, 0.6 g/kg of BCAA was consumed in the morning and afternoon after diluting it in water. The placebo was packaged to conceal the contents from the participants. A similar amount was diluted in orange juice and administered in the morning and afternoon at the same time as the BCAA. All participants were requested to maintain a diet journal during the experiment.
Blood analyses
Blood samples (10 ml) were collected between 8-9:00 am in the morning in a fasted state from the brachial vein. The collected samples were clotted for 30 min at room temperature and centrifuged for 10 min at 3,000 rpm to separate the serum. We stored the separated serum samples in a freezer at -90ºC. We used Modular Analytics (Roche, Germany) to analyze the blood CK, LDH, and AST concentrations.
Statistical analyses
The mean (M) and standard deviation (SD) were computed for each parameter using the SPSS software (version, 20). We conducted two-way analysis of variance with repeated measures to analyze the significance of the data according to the treatment and time of measurement. A post-hoc comparison was performed using independent and dependent t-tests according to the time of measurement. The statistical significance was set at P<.05. The Pearson’s correlation method was used to analyze the correlations between the peak torque and the indicators of muscle injury.
RESULTS
Knee extension and flexion peak torque
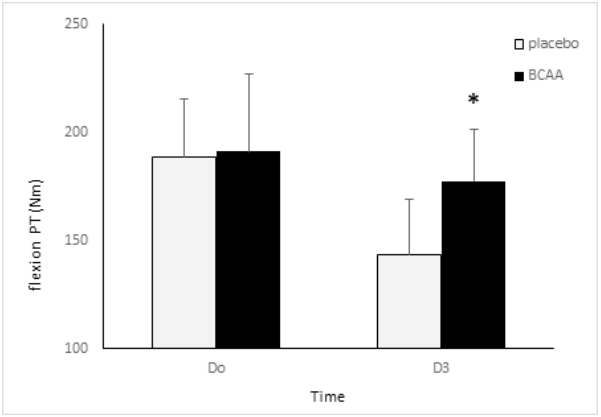
Figures 2 and 3 show the changes in knee extension and flexion peak torque between two groups. The placebo group demonstrated a significant reduction of the extension peak torque from 332.3±34 Nm at the baseline (D0) to 267.3±28 Nm during post-treatment (D3) (p<.05). The BCAA group displayed a reduction of the extension peak torque from 355.6±43 Nm at the baseline to 313.2±51 Nm at the post-treatment. However, the change was insignificant. There were significant differences between the groups. The post-hoc test revealed a significant reduction in the placebo group, compared to the BCAA group (p<.05). Regarding flexion peak torque, The placebo group revealed a significant reduction in the flexion peak torque from 188.3±27 Nm at the baseline (D0) to 143.2±26 Nm at post-treatment (D3) (p<.05). In contrast, the BCAA group displayed an insignificant reduction from 191.2±36 Nm at the baseline to 177.5±24 Nm at post-treatment.
Blood AST
Figure 4 shows the changes in blood AST between two groups. The placebo group showed a significant elevation in the AST concentration from 13.7±3.2 U/L before the exercise (pre-D0) to 25.1±3.8 U/L, 39.2±4.8 U/L, 48.4±6.7 U/L, and 36.3U±7.2 U/L immediately after (post-D0), and at 24 h (D1), 48 h (D2), and 72 h (D3) after the exercise, respectively (p<.05). Further tests showed that the AST levels had significantly increased after the exercise (post-D0, D1, D2, and D3), compared to the pre-exercise concentrations (pre-D0). In addition, the levels were significantly elevated at D2, compared to that at post-D0. The BCAA group revealed a substantial increase in the AST levels from15.2±3.1 U/L before the exercise (pre-D0) to 27.6±4.8 U/L, 37.5±6.8 U/L, 42.7±7.2 U/L, and 33.4±5.9 U/L immediately after (post-D0), and at 24 h (D1), 48 h (D2), and 72 h (D3) after the exercise, respectively (p<.05). Further tests showed that the AST levels had significantly increased after the exercise (post-D0, D1, D2, and D3), compared to the pre-exercise concentrations (pre-D0) (p<.05). Moreover, the levels were significantly elevated at D2, compared to that at post-D0 (p<.05). There were no significant differences between the groups.
Blood CK
Figure 5 shows the changes in blood CK between two groups. The CK concentration significantly and continuously increased in the placebo group from 157.3±23.8 U/L before the exercise (pre-D0) to 221.4±36.1±36.1 U/L, 281.7±42.8 U/L, 366.3±51.7 U/L, and 382.8±47.2 U/L immediately after (post-D0), and at 24 h (D1), 48 h (D2), and 72 h (D3) after the exercise, respectively (p<.05). Further tests showed that the CK levels had significantly increased after the exercise (post-D0, D1, D2, and D3), compared to the pre-exercise concentrations (pre-D0). In addition, the levels were significantly elevated at D1 and D2, compared to that at post-D0 (p<.05). The BCAA group displayed a continuous and significant increase in the CK concentrations from 141.3±17.1 U/L before the exercise (pre-D0) to 236.2±34.8 U/L, 289.3±36.8 U/L, 304.5±47.2 U/L, and 314.8±37.9 U/L immediately after (post-D0), and at 24 h (D1), 48 h (D2), and 72 h (D3) after the exercise, respectively (p<.05). Further tests showed that the CK levels had significantly increased after the exercise (post-D0, D1, D2, and D3), compared to the pre-exercise concentrations (pre-D0). Furthermore, the levels were significantly elevated at D1, D2, and D3, compared to that at post-D0 (p<.05). There were significant differences among the groups. The post-hoc comparison revealed substantially elevated CK levels in the placebo group at D2 and D3, compared to that in the BCAA group (p<.05).
Blood LDH
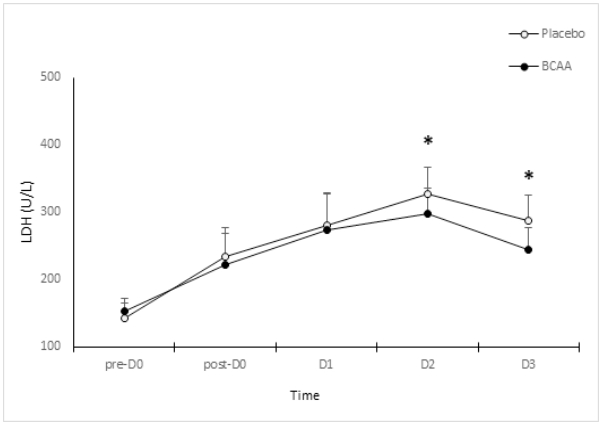
Figure 6 shows the changes in blood LDH between two groups. The placebo group revealed a significant increase in the LDH levels from 142.3±23U/L before exercise (pre-D0) to 234.4±43 U/L, 279.7±48 U/L, 326.3±41 U/L, and 287.8±37 U/L immediately after (post-D0), and at 24 h (D1), 48 h (D2), and 72 h (D3) after the exercise, respectively (p<.05). Further tests showed that the CK levels had significantly increased after the exercise (post-D0, D1, D2, and D3), compared to the pre-exercise concentrations (pre-D0). Furthermore, the levels were significantly elevated at D2, compared to that at post-D0 (p<.05). LDH levels in the BCAA group had significantly changed from 153.3±18U/L before the exercise (pre-D0) to 221.2±48 U/L, 273.3±56 U/L, 298.5±37 U/L, and 244.8±32 U/L immediately after (post-D0), and at 24 h (D1), 48 h (D2), and 72 h (D3) after the exercise, respectively (p<.05). Further tests showed that the CK levels had significantly increased after the exercise (post-D0, D1, D2, and D3), compared to the pre-exercise concentrations (pre-D0). Moreover, the levels were significantly elevated at D2, compared to that at pre-D0 (p<.05). There were significant differences between the groups. The post-hoc comparison revealed substantially elevated LDH levels at D2 and D3 in the placebo group, compared to that in the BCAA group (p<.05).
The correlation between the peak torque and indicators of muscle damage
Table 1 summarizes correlations between the extension and flexion peak torques and the levels of CK, LDH, and ASK in the BCAA group on the last day of measurement (D3).
There was no correlation between the extension and flexion peak torques. The extension peak torque was negatively correlated with the CK and LDH levels. In other words, the CK and LDH levels decreased with an increasing extension peak torque (p<.05). Furthermore, the flexion peak torque was negatively correlated with the LDH level. The latter decreased with an increasing flexion peak torque (p<.05). The CK and LDH levels were positively correlated, thus indicating the levels proportionately changed to each other (p<.05).
DISCUSSION
Both groups showed a significant reduction in the extension peak torque at the second isokinetic exercise (D3), compared to that at the baseline because of the DOMS. The BCAA group had a significantly higher value at D3, compared to the placebo group. Furthermore, both groups showed a significant reduction in the flexion peak torque at the D3, compared to that at D0. In addition, the BCAA group had a significantly higher value, compared to the placebo group. A prior study [23] reported on a substantial increase in the peak torque after inducing DOMS with resistance exercise in the BCAA group, compared to the placebo group. Furthermore, following the isokinetic exerciseinduced DOMS, the peak torque decreased until 96 h after the exercise [24]. There was a decrease in the extension peak torque until 72 hours after the DOMS-inducing exercise. The BCAA supplements inhibited the reduction of the peak torque during the aforementioned process. Our results support previous findings that BCAA administration can inhibit the exercise-mediated breakdown of muscle proteins [25]. Furthermore, Howatson et al. [26] mentioned that the action of BCAA in stimulating protein synthesis facilitates the quick recovery of muscle injury caused by resistance exercise. This was attributed to the BCAA-controlled inhibition of the breakdown of skeletal muscle proteins [18]. BCAA is particularly involved in escalated protein synthesis by stimulating the insulin-like growth factor 1. This eventually stimulates the mammalian target of rapamycin (mTOR) [27]. Therefore, BCAA administration after a vigorous exercise might promote protein synthesis during the post-exercise recovery phase. The extension and flexion peak torques were higher in the BCAA group before and after DOMS. This might be a consequence of an increased protein synthesis because of BCAA administration, which in turn inhibited the reduction of peak torque.
The AST tended to increase after the exercise (post-D0, D1, D2, and D3), compared to that before the exercise (pre-D0). Nonetheless, there were no significant differences between the two groups. The AST concentrations tended to increase until 48 h (D2) the after DOMS-inducing exercise. This supports previous findings that reported on significant elevations in ASK levels after a high-intensity training that induces muscle injury and muscle fatigue [28]. However, BCAA administration did not reduce the ASK levels. The AST levels particularly increased until (D2). However, they began to decline after D3, unlike the trends observed for the CK and LDH levels. A prior study reported on an increase in the blood ASK levels by more than seven-fold in liver cells compared to that in the skeletal muscles during a marathon [29]. Therefore, the ASK levels are more specific to liver cells than skeletal muscles in exercises the induce muscle injuries. In contrast, the CK and LDH concentrations tended to increase over time after a DOMS-inducing exercise. The BCAA group showed a significantly lower value of CK and LDH, compared to the placebo group. According to a previous study [1], the CK levels begin to increase 24 h after a vigorous exercise and peak at 48 h. Similarly, the CK and LDH levels significantly increased until 72 h (D3) after the exercise. Moreover, BCAA administration inhibited the increase of the CK and LDH levels. Our findings support the results of Greer et al. that BCAA administration inhibits muscle injuries caused by an endurance exercise by reducing the CK and LDH levels [20]. This was attributed to the action of BCAA in reducing muscle protein oxidation [30]. Rahimolou et al. [31] conducted a meta-analysis on BCAA and muscle injuries and stated that BCAA inhibits muscle injuries. Therefore, their continuous use would be effective for endurance athletes. Furthermore, a prior study [19] reported on a significant decline in the CK and LDH levels after administering BCAA for 7 days, followed by an endurance exercise. However, another study [32] stated that BCAA administration prior to resistance exercise did not change the CK levels. The inconsistent results are reportedly affected by the type of exercise, intensity, duration, recovery period, and the duration and amount of BCAA administration [33,34]. Exercise programs that may particularly induce DOMS can lead to different outcomes. There was no correlation between the extension and flexion peak torques. Therefore, BCAA administration did not affect the interaction between the agonistic and antagonistic muscles in the knee peak torque in a state of DOMS. In contrast, the extension peak torque was negatively correlated with the CK and LDH levels. In other words, the CK and LDH concentrations changed mutually and proportionately. The aforementioned levels decreased with an increasing extension peak torque. However, the LDH levels declined with an increasing flexion peak torque. In conclusion, while the peak torque decreases, the CK and LDH levels proportionately increase upon inducing DOMS by an exercise. However, BCAA inhibits the reduction of the extension peak torque and the elevation of CK and LDH levels during this process. Therefore, BCAA can be used as a supplement for athletes engaging in vigorous exercise. This might induce DOMS to maintain muscle strength and prevent muscle damage.