 |
 |
- Search
| Phys Act Nutr > Volume 27(4); 2023 > Article |
|
Abstract
[Purpose]
This review aimed to comprehensively explore and elucidate multifaceted neutrophils in breast cancer, particularly in the context of physical activity. Neutrophils play a critical role in the tumor microenvironment and systemic immune response, despite their short half-life and terminal differentiation. Through a thorough review of research related to changes in immunity in breast cancer during exercise, this review aims to provide comprehensive insights into immunological changes, especially focusing on neutrophils. Recognizing that much of the existing research has predominantly focused on T cells and nature killer (NK) cells, our review seeks to shift the spotlight toward understanding how exercise affects neutrophils, a less-explored but critical immune response component in breast cancer.
[Methods]
This study involved an extensive review of the literature (from 2000 to 2023) using the PubMed, Science Direct, and Google Scholar databases. The keywords chosen for the searches were “immune cells and exercise,” “exercise and breast cancer,” “tumor microenvironment and neutrophils,” and “neutrophils and exercise and breast cancers.”
[Results]
Neutrophils in the tumor microenvironment can exhibit distinct phenotypes and functions. These differences have yielded conflicting results regarding tumor progression. Exercise plays a positive role in breast cancer and alters the immune system. Physical activity can quantitatively and functionally regulate neutrophils under various conditions such as metabolic disruption or senescence.
Breast cancer is the most prevalent cancer in women worldwide. According to the International Agency for Research on Cancer (IARC), an estimated 2.3 million new breast cancer cases were diagnosed in 2020, accounting for approximately 11.7% of all new cancer cases. Although advances in breast cancer treatment have led to increased five-year survival rates, cancer recurrence remains a significant contributor to mortality [1].
Surgery is often the primary treatment for breast cancer; however, adjuvant therapies such as cytotoxic chemotherapy and hormone therapy are routinely employed to prevent recurrence. Despite their widespread use, these treatments have limitations in effectively reducing recurrence rates and can cause substantial side effects [2]. Given the high risk of breast cancer recurrence beyond the critical five-year threshold and the significant side effects associated with cytotoxic chemotherapy, there is an urgent need for more effective strategies to prevent cancer recurrence [3].
A recent breakthrough in immunotherapy, which engages the body’s natural immune system to fight cancer, heralded a transformative era of cancer therapy with improved patient outcomes [4,5]. Generally, lifestyle factors such as diet and physical activity are recognized for their beneficial effects on immune function [6,7]. Regular exercise has been shown to foster a robust immune response by promoting immune cell circulation and reducing inflammation, thus helping the body combat pathogens and abnormal cells, including those implicated in cancer [7]. Growing evidence suggests that exercise plays a beneficial role in reducing the risk of breast cancer recurrence and improving overall well-being [8]. Studies have shown that breast cancer survivors who engage in regular exercise have a lower cancer recurrence risk. Moreover, exercise can help mitigate the side effects of breast cancer treatment, thereby improving patients’ quality of life [8]. Individuals undergoing chemotherapy often experience fatigue as a side effect; however, regular exercise has been shown to counteract this fatigue and improve overall energy levels [9]. Exercise has been linked to the activation of natural killer (NK) cells, T cells, neutrophils, and other immune cells [6,10]. These cells are integral to the immune system’s capacity to identify and eradicate cancer cells and offer a complementary defense mechanism that supports traditional cancer therapies [5].
Neutrophils, known for their role in countering bacterial infections, are now being revealed to play a multifaceted role in cancer, particularly in the context of breast cancer, where they may contribute to both tumor progression and metastasis [11,12]. Neutrophils release various factors and enzymes that promote tumor growth and progression. They secrete cytokines, chemokines, growth factors, and matrix metalloproteinases, which contribute to angiogenesis (formation of new blood vessels), tumor cell migration, invasion, and tissue remodeling [11,12]. Neutrophils can interact with other immune and tumor-associated cells, forming a pro-tumor microenvironment [12]. They may exhibit immunosuppressive properties in the tumor microenvironment, contributing to immune surveillance evasion. They can release molecules such as arginases, reactive oxygen species (ROS), and immunosuppressive cytokines, which can inhibit the activity of cytotoxic T cells and NK cells, impairing the immune response against cancer cells [12].
This review aimed to comprehensively explore and elucidate the multifaceted role of neutrophils in breast cancer, particularly in the context of physical activity and exercise. Neutrophils play a critical role in the tumor microenvironment and systemic immune response, despite their short half-life and terminal differentiation. By comprehensively reviewing research on how exercise modulates immunity in breast cancer, this review aims to offer compelling insights into immunological changes, particularly those involving neutrophils. Recognizing that much of the existing research has predominantly focused on T cells and NK cells, our review seeks to shift the spotlight toward understanding how exercise affects neutrophils, a less-explored but equally critical immune response component in breast cancer.
This study involved an extensive review of the literature (from 2000 to 2023) using the PubMed, Science Direct, and Google Scholar databases. The keywords chosen for the searches were “immune cells and exercise,” “exercise and breast cancer,” “tumor microenvironment and neutrophils,” and “neutrophils and exercise and breast cancers.”
Breast cancer is the leading cause of cancer-related deaths in women worldwide. In 2020, the World Health Organization (WHO) reported 2.3 million new cases of breast cancer in women, tragically leading to 685,000 deaths. Additionally, an estimated 7.8 million women worldwide were living with breast cancer diagnosed within the past five years as of the end of 2020. While the exact etiology of breast cancer is complex and multifaceted, with factors ranging from genetic predisposition to lifestyle choices playing a role, it is broadly understood that sex, aging, hormonal influences, family history, and lifestyle factors such as diet and physical inactivity are associated with an increased risk of breast cancer development [13-15]. Although a few of these factors are inherent and unchangeable, lifestyle is a modifiable element with a significant preventative potential. Physical activity, which encompasses all movements that expend energy, has been identified as a key factor in reducing breast cancer risk.
Physical activity refers to all body movements related to skeletal muscles through energy expenditure, and physical exercise is a subcategory of planned, structural, and repetitive physical activity to improve or maintain physical strength [16,17]. A dose-response analysis by Dia et al. (2023) reported an inverse correlation between physical activity levels and cancer risk [18] and showed that 150-300 min of moderate-intensity exercise (or 75-150 min of vigorous exercise) per week can reduce the breast cancer risk by 25-30% compared to sedentary adults [19]. A meta-analysis of preclinical studies confirmed that exercise can reduce tumor burden and incidence in animal models of breast cancer, such as mice and rats [20]. Observational studies in humans have linked higher activity levels with reduced mortality and recurrence rates among patients with breast cancer patients [21-23]. Although chemotherapy, commonly used in breast cancer treatment, is effective, there is a high risk of drug resistance and other side effects. Exercise has been proposed as an effective method to alleviate the adverse effects of these treatments [24].
In general, decreased physical activity, negative changes in body composition, increased tendency toward depression or anxiety, and fatigue may occur during breast cancer treatment [17,25,26]. Women diagnosed with breast cancer tend to have reduced physical activity levels, with a much greater decline observed in treated patients than in untreated patients [27]. Additionally, a prospective cohort study demonstrated that only 28% of US cancer survivors engaged in the recommended physical activity, indicating that they remained inactive [28]. However, the benefits of regular exercise extend beyond physical health; studies have reported that it can alleviate anxiety and depression in women with early-stage breast cancer, contributing to enhanced psychological well-being [29]. In addition, regular exercise offsets fatigue in patients undergoing chemotherapy and improves energy levels, thereby improving the quality of life [25,30].
Therefore, exercise may contribute to breast cancer prevention, prognosis, and overall management. Consequently, there is a growing body of research exploring the benefits of physical exercise in individuals with breast cancer, both during and after treatment. Exercise has been shown to enhance various physiological and psychological aspects including cardiopulmonary health, muscle strength, and overall well-being, making it a vital area of study in cancer care.
Breast cancer is a tumor that forms in the breast tissue and mainly occurs in the milk ducts or lobules [31]. Non-invasive breast cancer which is also called ductal carcinoma in situ (DCIS) is a premalignant lesion that can develop into invasive breast cancer, in which cancer cells reside within the milk ducts but do not spread to healthy breast tissue. Invasive breast cancer, depending on the tissue and cell type, includes invasive ductal carcinoma (IDC), which is the most common form of breast cancer, accounting for over 80% of all cases, and invasive lobule carcinoma (ILC), which accounts for 5-15% of breast cancers and has a high incidence in older women [32]. Breast cancer develops through the accumulation of mutational changes and the progression and development of these conditions can be influenced by multiple factors in the ductal microenvironment [33]. Within this intricate network, immune cells play a pivotal role in modulating the dynamic interplay between the host immune system and the evolving neoplastic landscape.
Immune cell infiltrates in breast cancer comprise multiple cell subtypes, including cluster of differentiation 4+ (CD4+) cells, CD8+ cells, B cells, monocytes/macrophages, dendritic cells, and natural killer (NK) cells, which directly (CD4+ and CD8+ cell-mediated cytotoxicity) and indirectly (secreted cytokines, growth factors, and other agents) may influence tumor growth on tumor cells and other cells in the microenvironment. These immune cells, including T lymphocytes, macrophages, and dendritic cells, perform multifaceted functions ranging from tumor surveillance to immunomodulation. In particular, the innate immune system (granulocytes, natural killer cells, macrophages, dendritic cells, and monocytes) and adaptive system (lymphocytes, including T and B cells) enter the tumor microenvironment and act effectively to regulate the growth of tumor cells [34]. The concerted efforts of immune cells within the tumor microenvironment profoundly influence the cancer progression trajectory, thereby affecting therapeutic responses and clinical outcomes.
As the tumor progresses, the evolving immune microenvironment undergoes significant alterations, marked by a gradual decline in cytotoxic CD8+ T cells and NK cells, an increase in dysfunctional CD8+ T cells, and notable changes in immune regulatory components, such as immunosuppressive CD4+ Forkhead box P3 regulatory T (FoxP3+ Treg) cells and modulatory B cells. Myeloid cells, which are associated with tumors, actively facilitate tumor angiogenesis and enhance vascular permeability. For instance, TIE-2-expressing macrophages located within the perivascular niche (PVN) of tumors actively promote angiogenesis in various mouse tumor models. Therefore, managing the immune system may be an effective treatment strategy for breast cancer. In addition, cancer immunotherapy is rapidly changing the field of oncology.
Exercise clearly affects the immune response and is reported to affect the production of cytokines and signaling molecules involved in regulating immune cell function [35]. Exercise can activate specific immune cell pathways, particularly increasing the number and activity of T cells, which play an important role in targeting cancer cells, allowing them to better identify and attack cancer cells [36-38]. Rundqvist et al. (2020) reported that running exercise in breast cancer mice modified cytotoxic T cell metabolism and that the anti-tumor effect of exercise training disappeared when CD8+ T cells were depleted. This showed that the anti-tumor effect of exercise depends on CD8+ T cells and that exercise can change the intrinsic metabolism and anti-tumor effector function of cytotoxic T cells. This may play a role in promoting nascent T-cell responses, indicating that the adaptive immune system is a key component in exercise-induced tumorigenesis suppression [39].
Recently, quantification of the intratumoral ratio between CD8+ cytotoxic T cells and FoxP3+ Treg cells as an antitumor immune indicator indicated an ability to suppress the anti-tumor response, and a decrease in the ratio due to a high number of FoxP3+ Tregs was associated with poor prognosis in breast cancer patients. Running exercise that minimizes stress showed no difference in CD+ T cells in breast cancer animal models but improved anti-tumor immunity by decreasing FoxP3+ Tregs and increasing the CD8+/Foxp3+ratio [40].
Exercise increases the number and activity of NK cells, which is another pathway that can potentially destroy cancer cells more effectively [36,38,41]. NK cells are the most responsive immune cells to exercise, and it has recently been revealed that NK cell mobilization following exercise plays a key role in cancer prevention [42]. Exercise increases intratumoral vascularization and perfusion and promotes immune cell subsets to access the tumor site. Increased body temperature due to exercise improves the ability of the tumor vasculature to allow immune migration to the tumor. Therefore, NK cell mobilization and activation following exercise demonstrated protective effects against cancer, suggesting that exercise is a potential adjuvant therapy for cancer [42].
Cancer cells manipulate their surroundings by enlisting and altering non-cancerous cells and restructuring the vasculature and extracellular matrix (ECM) to create a supportive environment for tumor growth [43]. Neutrophils are the most abundant immune cells in the blood. Neutrophils play a major role in eliminating pathogens through various mechanisms [12]. They engulf and digest microorganisms through a process known as phagocytosis. Neutrophils release antimicrobial proteins and enzymes that destroy pathogens. The diversity and plasticity of tumor-associated neutrophils (TANs) are currently under intensive investigation in cancer research 44 . Neutrophils are recruited to the tumor microenvironment in certain situations, adopting a distinct phenotype known as TANs [44]. Depending on the signal and maturity status within the tumor microenvironment (TME), neutrophils can exhibit either anti-tumor or pro-tumor functions [44]. Their systemic accumulation contributes to immune suppression in distant organs and extracellular matrix remodeling, thereby promoting metastatic niche formation. Amy-Jo et al., (2015) revealed that tumor modification of hematopoiesis toward altering neutrophil output in the bone marrow occurs at the beginning of malignancy [45]. In addition, TANs that produce matrix metallopeptidase 9 (MMP-9) play a crucial role in driving angiogenesis in the pancreatic islet carcinogenesis model [12]. In addition, they suppress cytotoxic T-cell activity and promote immune evasion by tumors [12].
Neutrophils with protumorigenic characteristics are classified as N2 TANs. Researchers have suggested that N2 TANs may be similar to myeloid-derived suppressor cells (MDSCs) [12,45]. MDSCs constitute a heterogeneous bone marrow-derived cell population comprising immature mononuclear cells and neutrophils endowed with potent immunosuppressive capabilities [12]. These cells expand in patients with cancer and murine tumor models, and their presence in the tumor microenvironment is associated with unfavorable clinical outcomes. MDSCs exert their immunosuppressive effects on T, NK, B, and dendritic cells through various mechanisms, including the secretion of immunosuppressive factors and cell-to-cell contact mechanisms [12].
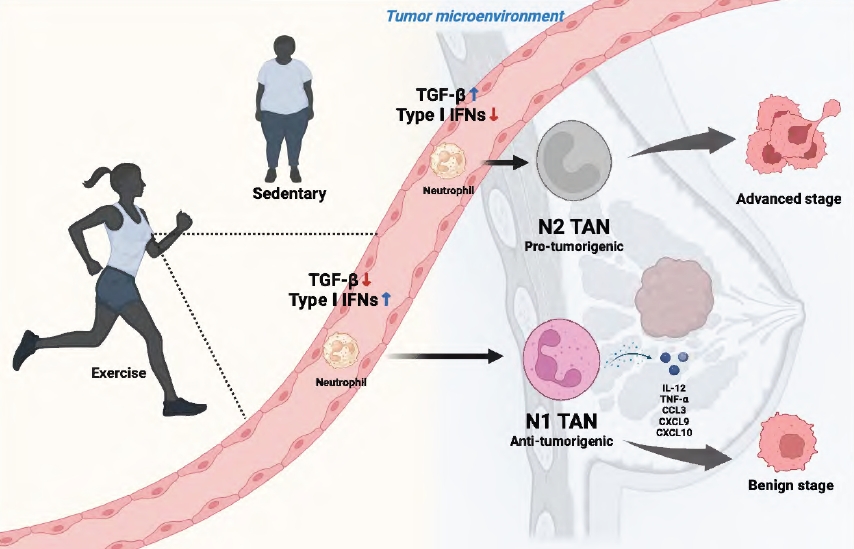
Certain preclinical experiments have demonstrated antitumor effects of neutrophils classified as N1 TANs [12,44,45]. N1 neutrophils exhibit robust anti-tumor activity, primarily attributed to their secretion of pro-inflammatory molecules such as interleukin (IL)-12, tumor necrosis factor (TNF)-α, and chemokines like C-C Motif Chemokine Ligand 3 (CCL3), C-X-C motif chemokine ligand 9 (CXCL9), and CXCL10. Several researches have demonstrated the pivotal involvement of the cytokine transforming growth factor-beta (TGF-β) in neutrophil polarization [12,44,45]. Exposure to TGF-β drives the development of neutrophils towards an N2 phenotype, while its inhibition favors the N1 phenotype. Additionally, type I interferons (IFNs) play a crucial role in differentiating and activating TANs towards the N1 phenotype.
Mishalian et al. (2013) reported that the total number of neutrophils infiltrating the tumor remained unchanged and that in the early tumor development stages, neutrophils were predominantly localized around the tumor [46]. In later stages, neutrophils were observed to be scattered among the tumor cells. Additionally, we observed that early-stage TANs exhibited increased cytotoxicity towards tumor cells and produced higher TNF-α, nitric oxide (NO), and hydrogen peroxide (H2O2) levels compared to later stages, that TANs have an N1 phenotype at an early stage.
In general, cancer cells tend to display elevated baseline ROS levels compared to normal cells owing to an imbalance between oxidants and antioxidants. Studies have demonstrated that TGF-β1 can enhance mitochondrial ROS generation in diverse cell types [47]. Above all, TGF-β is a factor that determines the fate of TANs as mentioned ahead [12,44,46].
Previous studies have shown that exercise affects TGF-β levels [48], which may be able to alter TANs to N2 type in the liver cancer mice model. The authors compared the effects of moderate swimming with overloaded swimming on cancer progression and survival rates in liver cancer-transplanted C57BL/6 mouse models. They observed that 8 min/d (moderate) swimming decreased TGF-β1 levels both in serum and liver cancer tissues. In humans, a study compared the level of TGF-β1 in the regular cyclist (2-3 times a week, average 30-60 min each) with sedentary control [49]. They observed that the level of TGF-β1 was lower in the recreational cyclist than in the sedentary control group. Several other studies have confirmed that participating in moderate regular exercise would reduce the level of circulating TGF-β in both human and rodents [49-51].
The type I IFN constitute IFN-α, IFN-β, IFN-ε, IFN-κ and IFN-ω in human. IFN was first discovered as a protein produced by infected cells in response to viral infections, and serves as a substance to counteract viruses [52]. The production of type I IFN is triggered in response to extensive damage, and the subsequent immediate response causes cell death 53 . And type I IFN is known to influence the functional direction of TANs [12,46]. Metastatic cancer partially responds to chemotherapy; therefore, various immunotherapies have been used as alternatives. IFN is emerging as a promising alternative therapy, with a particular focus on IFN-γ, which demonstrates significant anti-tumor effects. Although type I IFN plays an important role in cancer, studies investigating the effect of exercise on IFN α levels in cancer models are rare. However, one study investigated the tendency of the IFN family to be influenced by exercise in situations or models other than cancer. A study showed that continuous moderate-intensity exercise increased the level of IFN-γ secreted by peripheral blood mononuclear cells (PBMCs), which decreased after 2 months [54]. Additionally, if the exercise duration is too short or the exercise intensity is too high, the opposite effect may be seen [54]. Dynamic fluctuations in blood hormones, metabolites, myokines, adipokines, hepatokines, extracellular vesicles, and other factors occur during exercise, all of which may affect the function of neutrophils during cancer progression [55]. Considering the critical role of IFN in cancer, future research on the quantitative changes in blood IFN levels during exercise is needed. Particularly, it is expected that exercise intensity or time can affect changes in the amount of IFN; therefore, a more specific and detailed experimental design is required for further research.
The changes in neutrophil characteristics and tumor environment induced by exercise suggest the potential of exercise to positively affect tumor progression regulation (Figure 1).
Regular physical activity plays a pivotal role in preventing and inhibiting cancer progression. Numerous studies have attempted to explain the mechanisms of exercise in cancer prevention by boosting the immune system, including NK and regulatory T cells. However, there are still limitations to fully explaining the mechanisms involved. NK cells have restricted infiltration into solid tumors, and abnormal regulation of T cells may cause transient B-cell aplasia, cytokine release syndrome (CRS), and neurotoxicities that cause cerebral edema. This suggests that the complex interactions between exercise, the immune system, and cancer prevention may not be completely understood based on the current state of knowledge, and there is a need for clear immune indicators that reflect appropriate exercise responses or survival in patients with cancer. Particularly, neutrophils, whose characteristics vary depending on the tumor microenvironment, need to be studied in the future to understand the cancer prevention effects of exercise. Detailed research is needed to elucidate how quantitative and qualitative changes in neutrophils influence the occurrence, growth, and metastasis of exercise-induced cancers. Therefore, it is necessary to develop a tool to check the status of neutrophils before and after exercise to determine the conditions for personalized exercise prescriptions for cancer prevention or treatment.
Acknowledgments
This study was funded by the National Research Foundation (NRF) of Korea (NRF-2022R1I1A4053049) and was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2022R1A5A8019303).
Figure 1.
Schematic diagram of the potential role of neutrophils mediating the anti-cancer effects of exercise on breast cancer malignancy. The modulation of TGF-β and type I IFN during exercise may influence TAN polarization, tipping the balance towards either an anti-tumorigenic (N1) or pro-tumorigenic (N2) phenotype.
REFERENCES
1. Kashyap D, Pal D, Sharma R, Garg VK, Goel N, Koundal D, Zaguia A, Koundal S, Belay A. Global increase in breast cancer incidence: risk factors and preventive measures. Biomed Res Int 2022;2022:9605439.




2. Scharl A, Salterberg A. Significance of ovarian function suppression in endocrine therapy for breast cancer in pre-menopausal women. Geburtshilfe Frauenheilkd 2016;76:516-24.



3. Riggio AI, Varley KE, Welm AL. The lingering mysteries of metastatic recurrence in breast cancer. Br J Cancer 2021;124:13-26.



5. Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med 2016;14:73.




6. Yoon KJ, Ahn A, Park SH, Kwak SH, Kwak SE, Lee W, Yang YR, Kim M, Shin HM, Kim HR, Moon HY. Exercise reduces metabolic burden while altering the immune system in aged mice. Aging (Albany NY) 2021;13:1294-313.



7. Shao T, Verma HK, Pande B, Costanzo V, Ye W, Cai Y, Bhaskar LVKS. Physical activity and nutritional influence on immune function: an important strategy to improve immunity and health status. Front Physiol 2021;12:751374.



8. Xu Y, Rogers CJ. Physical activity and breast cancer prevention: possible role of immune mediators. Front Nutr 2020;7:557997.



9. Rogers LQ, Vicari S, Trammell R, Hopkins-Price P, Fogleman A, Spenner A, Rao K, Courneya KS, Hoelzer KS, Robbs R, Verhulst S. Biobehavioral factors mediate exercise effects on fatigue in breast cancer survivors. Med Sci Sports Exerc 2014;46:1077-88.



10. Netea MG, Dominguez-Andres J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, Joosten LAB, van der Meer JWM, Mhlanga MM, Mulder WJM, Riksen NP, Schlitzer A, Schultze JL, Stabell Benn C, Sun JC, Xavier RJ, Latz E. Defining trained immunity and its role in health and disease. Nat Rev Immunol 2020;20:375-88.




11. Zheng C, Xu X, Wu M, Xue L, Zhu J, Xia H, Ding S, Fu S, Wang X, Wang Y, He G, Liu X, Deng X. Neutrophils in triple-negative breast cancer: an underestimated player with increasingly recognized importance. Breast Cancer Res 2023;25:88.




12. Xiong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol 2021;14:173.




13. Ataollahi MR, Sharifi J, Paknahad MR, Paknahad A. Breast cancer and associated factors: a review. J Med Life 2015;8:6-11.
14. Majeed W, Aslam B, Javed I, Khaliq T, Muhammad F, Ali A, Raza A. Breast cancer: major risk factors and recent developments in treatment. Asian Pac J Cancer Prev 2014;15:3353-8.


15. Sun YS, Zhao Z, Yang ZN, Xu F, Lu HJ, Zhu ZY, Shi W, Jiang J, Yao PP, Zhu HP. Risk factors and preventions of breast cancer. Int J Biol Sci 2017;13:1387-97.



16. Dasso NA. How is exercise different from physical activity? a concept analysis. Nurs Forum 2019;54:45-52.



17. García-Chico C, López-Ortiz S, Peñín-Grandes S, Pinto-Fraga J, Valenzuela PL, Emanuele E, Ceci C, Graziani G, Fiuza-Luces C, Lista S, Lucia A, Santos-Lozano A. Physical exercise and the hallmarks of breast cancer: a narrative review. Cancers 2023;15:324.



18. Diao X, Ling Y, Zeng Y, Wu Y, Guo C, Jin Y, Chen X, Feng S, Guo J, Ding C, Diao F, Du Z, Li S, Qiu H. Physical activity and cancer risk: a dose‐response analysis for the global burden of disease study 2019. Cancer Commun 2023;43:1229-43.

19. Kushi LH, Doyle C, McCullough M, Rock CL, Demark‐Wahnefried W, Bandera EV, Gapstur S, Patel AV, Andrews K, Gansler T. American cancer society guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin 2012;62:30-67.


20. Li Y, Xiao X, Zhang Y, Tang W, Zhong D, Liu T, Zhu Y, Li J, Jin R. Effect of exercise on breast cancer: a systematic review and meta-analysis of animal experiments. Front Mol Biosci 2022;9:843810.



21. Cannioto RA, Hutson A, Dighe S, McCann W, McCann SE, Zirpoli GR, Barlow W, Kelly KM, DeNysschen CA, Hershman DL, Unger JM, Moore HCF, Stewart JA, Isaacs C, Hobday TJ, Salim M, Hortobagyi GN, Gralow JR, Albain KS, Budd GT, Ambrosone CB. Physical activity before, during, and after chemotherapy for high-risk breast cancer: relationships with survival. J Natl Cancer Inst 2021;113:54-63.



22. Palesh O, Kamen C, Sharp S, Golden A, Neri E, Spiegel D, Koopman C. Physical activity and survival in women with advanced breast cancer. Cancer Nurs 2018;41:E31-8.



23. Patel AV, Friedenreich CM, Moore SC, Hayes SC, Silver JK, Campbell KL, Winters-Stone K, Gerber LH, George SM, Fulton JE, Denlinger C, Morris GS, Hue T, Schmitz KH, Matthews CE. American college of sports medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc 2019;51:2391-402.



24. Cormie P, Zopf EM, Zhang X, Schmitz KH. The impact of exercise on cancer mortality, recurrence, and treatment-related adverse effects. Epidemiol Rev 2017;39:71-92.


25. Darvishi E, Musarezaie A, Bahrami M, Karimian J. The effect of a combined exercise program on the fatigue severity of patients with breast cancer undergoing chemotherapy: a randomized clinical trial study. Iran J Nurs Midwifery Res 2023;28:398-404.


26. Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, von Gruenigen VE, Schwartz AL. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 2010;42:1409-26.

27. Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, Kriska A, Ballard‐Barbash R. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer 2003;97:1746-57.


28. Cao C, Friedenreich CM, Yang L. Association of daily sitting time and leisure-time physical activity with survival among US cancer survivors. JAMA Oncol 2022;8:395-403.



29. Vehmanen L, Mattson J, Karademas E, Oliveira-Maia AJ, Sousa B, Pat-Horenczyk R, Mazzocco K, Simos P, Cardoso F, Pettini G, Marzorati C, Kolokotroni E, Stamatakos G, Frasquilho D, Poikonen-Saksela P. Associations between physical exercise, quality of life, psychological symptoms and treatment side effects in early breast cancer. Breast J 2022;2022:9921575.




30. Adams-Campbell LL, Hicks J, Makambi K, Randolph-Jackson P, Mills M, Isaacs C, Dash C. An 8-week exercise study to improve cancer treatment related fatigue and QOL among African American breast cancer patients undergoing radiation treatment: a pilot randomized clinical trial. J Natl Med Assoc 2023;115:199-206.


31. Feng Y, Spezia M, Huang S, Yuan C, Zeng Z, Zhang L, Ji X, Liu W, Huang B, Luo W, Liu B, Lei Y, Du S, Vuppalapati A, Luu HH, Haydon RC, He TC, Ren G. Breast cancer development and progression: risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis 2018;5:77-106.



32. Swaminathan H, Saravanamurali K, Yadav SA. Extensive review on breast cancer its etiology, progression, prognostic markers, and treatment. Med Oncol 2023;40:238.



33. Goff SL, Danforth DN. The role of immune cells in breast tissue and immunotherapy for the treatment of breast cancer. Clin Breast Cancer 2021;21:E63-73.


34. Mukherjee AG, Wanjari UR, Namachivayam A, Murali R, Prabakaran D, Ganesan R, Renu K, Dey A, Vellingiri B, Ramanathan G, Doss CGP, Gopalakrishnan AV. Role of immune cells and receptors in cancer treatment: an immunotherapeutic approach. Vaccines 2022;10:1493.



35. Małkowska P, Sawczuk M. Cytokines as biomarkers for evaluating physical exercise in trained and non-trained individuals: a narrative review. Int J Mol Sci 2023;24:11156.



36. Forte P, Branquinho L, Ferraz R. The relationships between physical activity, exercise, and sport on the immune system. Int J Environ Res Public Health 2022;19:6777.



38. Lavín-Pérez AM, Collado-Mateo D, Abbasi S, Ferreira-Júnior JB, Hekmatikar AHA. Effects of exercise on immune cells with tumor-specific activity in breast cancer patients and survivors: a systematic review and meta-analysis. Support Care Cancer 2023;31:507.

39. Rundqvist H, Veliça P, Barbieri L, Gameiro PA, Bargiela D, Gojkovic M, Mijwel S, Reitzner SM, Wulliman D, Ahlstedt E, Ule J, Ostman A, Johnson RS. Cytotoxic T-cells mediate exercise-induced reductions in tumor growth. Elife 2020;9:e59996.




40. Hagar A, Wang Z, Koyama S, Serrano JA, Melo L, Vargas S, Carpenter R, Foley J. Endurance training slows breast tumor growth in mice by suppressing Treg cells recruitment to tumors. BMC Cancer 2019;19:536.




41. Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev 2000;80:1055-81.


42. Idorn M, Hojman P. Exercise-dependent regulation of NK cells in cancer protection. Trends Mol Med 2016;22:565-77.


43. Walsh LA, Quail DF. Decoding the tumor microenvironment with spatial technologies. Nat Immunol 2023;24:1982-93.



44. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009;16:183-94.



45. Casbon AJ, Reynaud D, Park C, Khuc E, Gan DD, Schepers K, Passegue E, Werb Z. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc Natl Acad Sci U S A 2015;112:E566-75.



46. Mishalian I, Bayuh R, Levy L, Zolotarov L, Michaeli J, Fridlender ZG. Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer Immunol Immunother 2013;62:1745-56.



47. Liu RM, Desai LP. Reciprocal regulation of TGF-beta and reactive oxygen species: a perverse cycle for fibrosis. Redox Biol 2015;6:565-77.


48. Zhang QB, Zhang BH, Zhang KZ, Meng XT, Jia QA, Zhang QB, Bu Y, Zhu XD, Ma DN, Ye BG, Zhang N, Ren ZG, Sun HC, Tang ZY. Moderate swimming suppressed the growth and metastasis of the transplanted liver cancer in mice model: with reference to nervous system. Oncogene 2016;35:4122-31.



49. Widiastuti IAE, Arsyad A, Idris I, Patellongi I, Kadriyan H, Buanayuda GW, Sari DP, Rosyidi RM. Exercise adaptations and TGF-beta1 levels in recreational cyclists. Ann Med Surg (Lond) 2021;70:102872.


50. Syahputra M, Lindarto D, Ramayani OR, Machrina Y, Purba A, Putra IB, Nasution IPA, Harahap J. Effect of moderate intensity continuous training and slow type interval training to gene expression of TGF-beta in type 2 diabetes mellitus model wistar rats. Med Arch 2023;77:4-7.



51. Kumar P, Stiernborg M, Fogdell-Hahn A, Mansson K, Furmark T, Berglind D, Melas PA, Forsell Y, Lavebratt C. Physical exercise is associated with a reduction in plasma levels of fractalkine, TGF-beta1, eotaxin-1 and IL-6 in younger adults with mobility disability. PLoS One 2022;17:e0263173.



52. Katze MG, He Y, Gale Jr M. Viruses and interferon: a fight for supremacy. Nat Rev Immunol 2002;2:675-87.



53. Jorgovanovic D, Song M, Wang L, Zhang Y. Roles of IFN-gamma in tumor progression and regression: a review. Biomark Res 2020;8:49.


54. Zamani A, Salehi I, Alahgholi-Hajibehzad M. Moderate exercise enhances the production of interferon-gamma and interleukin-12 in peripheral blood mononuclear cells. Immune Netw 2017;17:186-91.




55. Chow LS, Gerszten RE, Taylor JM, Pedersen BK, van Praag H, Trappe S, Febbraio MA, Galis ZS, Gao Y, Haus JM, Lanza IR, Lavie CJ, Lee CH, Lucia A, Moro C, Pandey A, Robbins JM, Stanford KI, Thackray AE, Villeda S, Watt MJ, Xia A, Zierath JR, Goodpaster BH, Snyder MP. Exerkines in health, resilience and disease. Nat Rev Endocrinol 2022;18:273-89.




- TOOLS






