Exercise-mediated macrophage polarization modulates the targeted therapeutic effect of NAFLD: a review
Article information
Abstract
[Purpose]
This review aims to explore the exercise-mediated hepatic macrophage polarization mechanism and its effect on improving and regulating non-alcoholic fatty liver disease (NAFLD) by analyzing the pathogenesis of NAFLD and the cause of the influence of hepatic macrophage polarization. In addition to exploring the varied effects of different exercise types on macrophage polarization regulation in NAFLD, to provide a direction and basis for the treatment of NAFLD.
[Methods]
The research methodology involved a comprehensive search of the PubMed database using specific keywords such as “NAFLD”, “macrophage polarization”, and “exercise”, to retrieve relevant literature published.
[Results]
(1) The main factors inducing NAFLD were high-fat diet, obesity, insulin resistance (IR), changes in gut microbiota, and genetic variation in susceptibility. (2) Drug treatment, nutrient induction, microfactor induction, physiological environment induction, and other factors can induce the polarization of hepatic macrophages and affect NAFLD. (3) Different intensities, types, and frequencies of exercise have different effects on polarization macrophages, and may also differently effects improving liver inflammation, fibrosis, and NAFLD. Curently, regular moderate-intensity aerobic exercise is the most effective therapy for treating NAFLD.
[Conclusion]
Approaches to ameliorate NAFLD with exercise involve strategies to alter macrophage polarization by inhibiting M1 or driving M2 activation. However, research on the different types of exercise-mediated macrophage polarization mechanisms and differences in therapeutic effects is not yet sufficient. Future research is necessary to explore the exact mechanisms and differences in the effects of different exercises on the treatment of NAFLD.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is a clinicopathological syndrome associated with obesity, inflammation, and insulin resistance. It is characterized by excessive lipid deposition in hepatocytes, with over 5% fat accumulation. NAFLD is broadly divided into two subtypes: non-alcoholic fatty liver disease (NAFL) (non-progressive NAFLD) and NASH(progressive NAFLD) [1,2]. The diagnosis of NASH requires histological evaluation in the setting of suspected NAFLD, typically characterized by steatosis, lobular inflammation, and ballooning, with or without perisinus fibrosis (Figure 1). Some individuals with NASH may develop progressive liver damage, cirrhosis, and HCC [3]. NAFLD affects approximately 30% of the world’s population. As the obese population continues to increase, there has been a search for interventions to treat and alleviate nonalcoholic fatty liver disease.
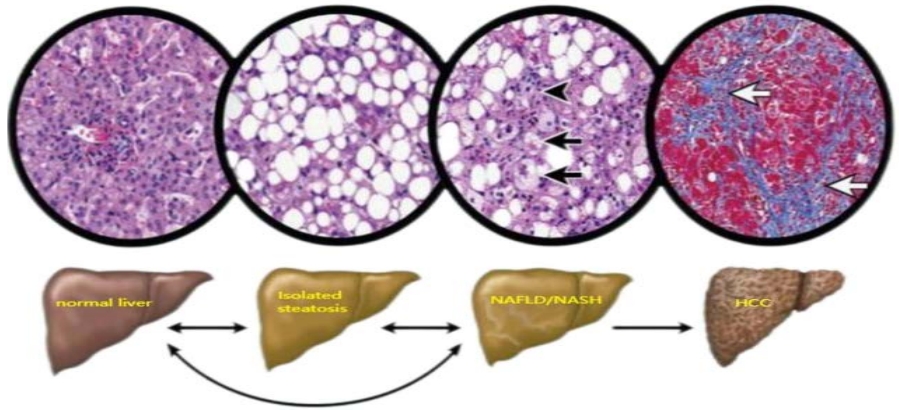
Nonalcoholic fatty liver disease (NAFLD) spectrum. Black arrows represent steatosis, inflammation, and ballooning of hepatocytes associated with varying degrees of liver fibrosis; white arrows represent collagen bands surrounding hepatic nodules that are characteristic of cirrhosis and other conditions. The different stages of the disease can regress or progress, as indicated by the arrows in the middle panel [3].
As an important part of the innate immune system, macrophages are indispensable in many aspects such as immune defense, inflammatory response, tissue remodeling and homeostasis.
Macrophage polarization plays a crucial role in physiological processes such as inflammation, tumors, tissue repair, and metabolism [4]. Macrophage polarization is closely associated with development and reversal of NAFLD. Targeting macrophage polarization to block or reverse changes in NAFLD is considered a potential strategy for treating of liver diseases.
Among the many factors that affect polarization macrophages, the way mediated by the movement mechanism is undoubtedly the most important. Exercise has many advant over other methods for treating NAFLD. First, compared to drugs that change the polarization of hepatic macrophages to treat NAFLD, there are potential side effects and risks, such as drug-induced liver damage and, lung function damage, In contrast, exercise therapy and improvement of NAFLD are safer. Second, exercise affects NAFLD in several ways, including improving insulin sensitivity, regulating lipid metabolism, reducing fat accumulation in the liver, and reducing inflammation. Compared with single drug therapy, exercise can comprehensively regulate multiple pathological processes and provide a more comprehensive therapeutic effect. Simultaneously, as a lifestyle change, exercise is characterized by long-term sustainability. Good health can be maintained by maintaining moderate exercise, preventing the recurrence and progression of NAFLD. Finally, exercise not only improves NAFLD, but also positively impacts overall health. Moderate exercise can improve cardiorespiratory function, enhance muscle strength, improve metabolic processes, control weight, and help prevent and treat many chronic diseases; therefore, exercise therapy is the most commonly recommended treatment for patients with NAFLD.
As the mechanisms of NAFLD and macrophage polarization are very complicated, the process by which exercise promotes macrophage polarization and its role and mechanism in liver diseases must be further studied and elucidated.
This review aims to explore the mechanism of exercise-mediated hepatic macrophage polarization and its effect on improving and regulating NAFLD by analyzing the pathogenesis of NAFLD and the cause of the influence of hepatic macrophage polarization. We also explored the different effects of different exercise types on the regulation of macrophage polarization in NAFLD to provide direction and basis for research on the mechanism of exercise-mediated macrophage polarization and the treatment of NAFLD.
METHODS
Literature review
The research methodology involved a comprehensive search of the PubMed database using specific keywords such as “NAFLD”, “macrophage polarization”, and “exercise/training”, to retrieve relevant literature published before March, 2023. The obtained literature was sorted and classified into three parts: the pathological mechanism of NAFLD, the effect of hepatic macrophage polarization on NAFLD, and the effect of exercise on macrophage polarization, which were comprehensively sorted out the corresponding parts according to their needs.
RESULTS
Pathological mechanism of NAFLD
NAFLD is characterized by widespread liver changes ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma. The pathogenesis of NAFLD/NASH is complex and involves lipid accumulation, insulin resistance, inflammation, and fibrosis [5].
Factors that contribute to the pathogenesis of NAFLD (Figure 2) include high-fat diet, obesity, insulin resistance (IR), changes in the gut microbiota, and genetic variants for susceptibility [3]. A complex interplay of these factors leads to disturbances in lipid homeostasis and excessive accumulation of triglycerides and other lipid species in hepatocytes.
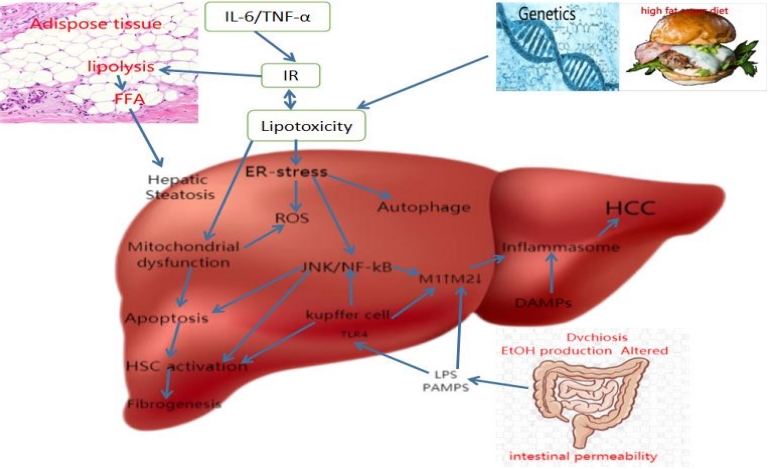
Schematic summary of NAFLD pathogenesis. Abbreviations: DAMPs, damage-associated molecular patterns; ER, endoplasmic reticulum; EtOH, ethanol; FFAs, free fatty acids; HCC, hepatocellular carcinoma; HSC, hepatic stellate cell; IR, insulin resistance; LPS, lipopolysaccharide; NAFLD, nonalcoholic fatty liver disease; PAMPs, pathogen-associated molecular patterns; ROS, reactive oxygen species; TLR4, Toll-like receptor 4.
Obesity with a high-fat diet genetically induced adipose tissue expansion, and IR-induced enhanced lipolysis lead to lipotoxicity-induced ER stress and mitochondrial dysfunction, then leading to ROS generation, liver cell autophagy, and activation of inflammatory pathway JNK/NF-kB. This causes hepatic macrophages to polarize and activate the M1 type, triggering chronic inflammation of the liver and NAFLD. At the same time, liver cell injury and death will also activate the activation of hepatic stellate cells, resulting in liver fibrosis [3].
Alterations in the balance of gut microbiota (i.e., dysbiosis) are also essential factors. Lipopolysaccharide (LPS), the main component of gut bacteria, plays a crucial role in liver inflammation and macrophage polarization during NAFLD. When the colonic mucosal immune function is impaired due to intestinal dysbiosis, LPS is no longer confined to the intestinal lumen but reaches the liver. Toll-like receptor 4 (TLR-4) on the plasma membrane of liver-resident cells recognizes LPS as a ligand that promotes receptor dimerization and subsequent activation of signaling cascades. Next, the LPS/TLR-4 cascade induces the production of classical inflammatory cytokines, including tumor necrosis factor-(TNF-α), interleukin-(IL-1β), and IL-6, which exacerbate the hepatic inflammatory state and promote fibrosis.
NAFLD/NASH is a complex disease in involving hepatic macrophages in multiple pathophysiological processes. Hepatic macrophages play critical roles in the progression of NAFLD. They can suppress liver inflammation and promote repair and regeneration by regulating the polarization state of hepatic macrophages.
Effect of hepatic macrophage polarization on NAFLD
Hepatic macrophages mainly exist in three subpopulations of different origins: yolk sac-derived tissue-resident macrophages-(KCs), monocyte-derived macrophages (MDM)/bone marrow-derived mononuclear macrophages, and liver capsule macrophages (LCMs) [6]. Among these, KCs are the most abundant type of tissue-resident macrophages in mammals, accounting for 80-90% of the total number of tissue-resident macrophages7. They are located within the hepatic sinusoids, receiving portal blood from the intestine and spleen, and arterial blood from the aorta. Therefore, KCs are critical sentinels at the interface between the liver and other organs.
Polarization of hepatic macrophages (Kupffer cells), that is, in a specific microenvironment, will show different functions and phenotypes. Hepatic macrophages are divided into two polarization states, M1 (classically activated macrophage) type and M2 (or activated macrophage) type. The polarization of M1-type hepatic macrophages is mainly triggered by stimulation of Th1-type cytokines (such as IFN-γ) and LPS, showing pro-inflammatory, pro-oxidative stress, pro-fibrosis, etc. develop. The polarization of M2 hepatic macrophages is mainly triggered by the stimulation of Th2 cytokines (such as IL-4 and IL-13), showing anti-inflammatory, anti-oxidative stress, and anti-fibrosis effects. It also helps to the reduce liver inflammatory Response and development of NAFLD.
As hepatic macrophage polarization is closely related to the development of NAFLD, factors that can participate in the intervention of hepatic macrophage polarization are also potential strategies for treating of NAFLD.
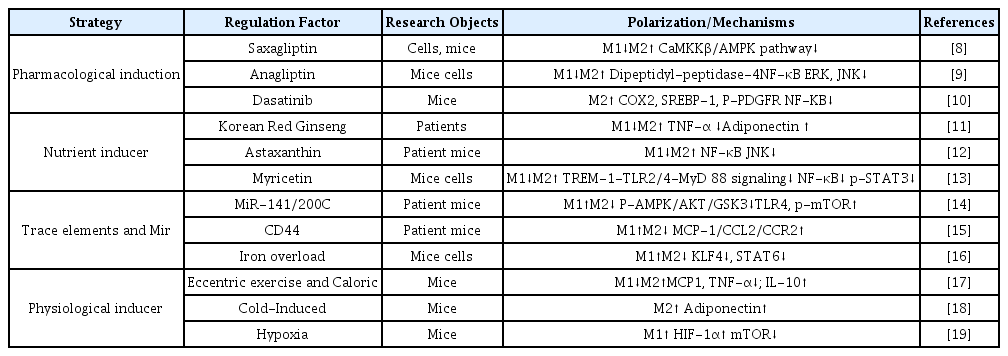
Polarization of hepatic macrophages is affected by a various factors (Table 1), including drug treatment, nutrient induction, microfactor induction of the physiological environment.
Many drugs have been shown to change the polarization state of hepatic macrophages. For example, Saxagliptin, Anagliptin, and Dasatinib, reduce M1 polarization and promote M2 polarization of hepatic macrophages through different molecular mechanisms. These drugs can improve NAFLD while activating the polarization of hepatic macrophages [8-10].
Some nutrients in the diet have also been shown to promote the polarized state of liver giant cells, such as: Korean Red Ginseng, Astaxanthin Myricetin, and others reduce inflammation and improve NAFLD through NF-κB/JNK/P38-MAPK and other signaling mechanisms [11-13].
Studies have confirmed that some tiny factors can also affect the polarization of hepatic macrophages. For example, MiR-141/200C, CD44, Iron overload, etc, have been confirmed to promote the polarization of hepatic macrophages to the M1 type [14-16]. In the research model, both NAFLD patients and experimental mice showed increased steatosis, liver damage and inflammation, which are the hallmarks of NAFLD.
Physiologically induced changes in the living environment are also necessary means of activating the polarization of hepatic macrophages. Eccentric exercise and Caloric increase anti-inflammatory IL-10 and inhibit pro-inflammatory MCP1, TNF-α, IL-1β, and IL-6 to reduce inflammation in serum and liver, promote hepatic M2 macrophage phenotype, inhibit M1 macrophage Phage cells, reduce chronic inflammation, improve hepatic steatosis [17]. Cold-induced fat accumulation can also be enhanced by promoting M2 type polarization [18]. In the hypoxic state, mice have increased ER stress detected in Kupffer cells (KCs), which promotes inflammation, simultaneously, hepatic macrophages shift to the M1 type [19].
Effects of exercise on macrophage polarization
Exercise can significantly affect the polarization of macrophages. During exercise, an increase in cellular metabolic activity and, changes in inflammatory factors and cytokines, affect the polarization state of macrophages. The metabolic activity of macrophages improves their intracellular environment, thereby promoting the polarization of M2 macrophages. Exercise promotes fatty acid oxidation and reduces fatty acids accumulation, reducing liver inflammation.
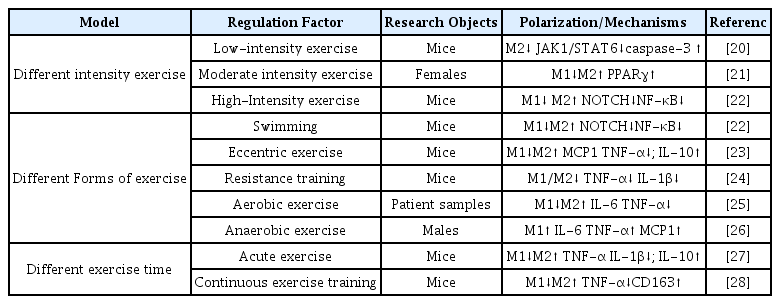
Current studies have confirmed that different types of exercise can promote the polarization state of macrophages. However there are few studies on the effects of different kinds of activity on the polarization state of hepatic macrophages. More research is needed to explore impact of different types of exercise on the differentiation of macrophage polarization. As shown in Table 2, activities ware divided according to intensity, type, and duration.
At different intensities, low-intensity exercise activates caspase-3 in female BALB/c mice to induce apoptosis, and reduce the polarization of M2 macrophages by inhibiting the JAK-STAT signaling pathway, thereby inducing tumor cell apoptosis and reducing tumor volume [20]. Participants in moderate intensity exercise may trigger signaling through PPARγ to suppress M1 markers and induce M2 markers in monocytes, contributing to improved systemic insulin sensitivity in exercise participants [21]. High-intensity exercise reversed serum biochemical parameters (glucose, triglyceride, cholesterol, insulin resistance homeostasis model assessment, and hsCRP) in male Wistar rats. Decreased M1 polarization markers (circulating IL-6, TNF-α and adipose tissue IL-6 mRNA, TNF-α and iNOS) and increased M2 markers (CD206, CD163, and IL10) expression [22].
There are currently studies on Swimming, Eccentric exercise, resistance training, and aerobic exercise among the different types of exercises. The decrease of M1 polarization markers (TNF-α, IL-1β) and the increase of M2 markers (IL-10) in the exercise state can indicate that different types of exercise can improve metabolism and chronic inflammatory response in humans or rodents, With significant improvement in symptoms of obesity-related insulin resistance, T2DM, and NAFLD [22-25]. Anaerobic exercises are a combination of high-intensity and short-duration, intermittent exercises. According to research, anaerobic exercise can significantly increase IL-6, IL-8, IL-10, TNF-α, and MCP1, polarize macrophages to the M1 type, leading to an intensified inflammatory response [26].
Acute exercise improves insulin signaling in the WAT fraction of Wistar rats and phenotypic transition from M1 to M2 macrophages in obese rats during exercise of different durations [27,28]. During continuous exercise training, male C57BL/6 mice not only inhibited the infiltration of M1 macrophages into adipose tissue, but also induced the phenotypic conversion of M1 macrophages to M2 macrophages in obese adipose tissue. Long-term exercise may inhibit adipose tissue inflammation by down-regulating TLR4 [28].
Mechanisms and targets of exercise-mediated macrophage polarization in the treatment of NAFLD
The signaling pathway of exercise-mediated macrophage polarization during the NAFLD treatment is complex and, involves multiple pathways and factors. The following are some classic signaling pathways(Figure 3):
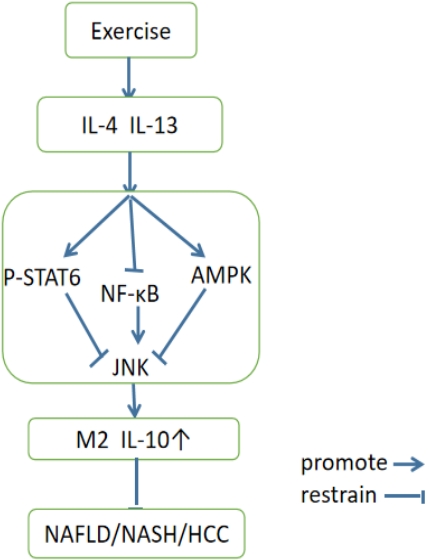
Mechanism of exercise-mediated macrophage polarization in NAFLD. Interleukin IL-4 and IL-13 are related cytokines that regulate many aspects of allergic inflammation. In macrophages, IL-4 and IL-13 induce alternative macrophage activation. AMP-activated protein kinase (AMPK) is a central regulator of energy homeostasis. Nuclear factor-κB (NF-κB) represents a family of inducible transcription factors, which regulates a large array of genes involved in different processes of the immune and inflammatory responses. c-Jun N-terminal kinases (JNKs), were originally identified as kinases that bind and phosphorylate c-Jun on Ser-63 and Ser-73 within its transcriptional activation domain.
(1) AMPK pathway: AMPK is an energy sensor. Exercise inhibits JNK by activating the AMPK pathway, which activates the M2 polarization of macrophages, thereby reducing inflammation, oxidative stress, and other mechanisms that alleviate the pathological process of NAFLD.
(2) JNK pathway: JNK (c-Jun N-terminal kinase(JNK) is a protein kinase that can participates in various cell signal transduction pathways. Lipotoxicity (lipotoxicity), plays avital role in these processes. Lipotoxicity is an essential mechanism for the occurrence and development of NAFLD.
(3) NF-κB pathway: Exercise can inhibit the activity of the NF-κB pathway, thereby reducing the inflammatory response and promoting the polarization of M2 macrophages, alleviating the pathological process of NAFLD.
Overall, exercise can promote the polarization of M2 macrophages by activating signaling pathways such as AMPK, JNK, and NF-κB pathways, thereby alleviating the pathological process of NAFLD. These pathways may interact with each other; however their specific mechanisms require further research.
Exercise-mediated macrophage polarization improves the condition of patients with NAFLD. However, there are differences in the polarization of hepatic macrophages under different exercise intensities, types, and frequencies, which may also effect liver inflammation, fibrosis, and the degree of NAFLD differently. Therefore, finding a more suitable exercise method can improve the pathological state of NAFLD.
At different exercise intensities, high-intensity exercise increased the accumulation of lactic acid in muscles, increased the production of free radicals and oxidative stress, increased the production of inflammatory factors, and promoted the polarization of M1 macrophages. During low-to-medium-intensity exercise, the concentration of lactic acid produced by the body is low, and the inflammatory response in the body is weak. It regulates lipid metabolism in liver cells, reduces fat accumulation and inflammatory response, and thus promotes the polarization of M2 macrophages. According to studies, high local concentrations of lactic acid and acute inflammation can regulate the metabolic mechanism of macrophages, making them polarize to the pro-inflammatory M1 type, In contrast lactic acid concentrations of and acute inflammation are more polarized to the M2 type [29,30]. Therefore, for patients with NAFLD, moderate and low-intensity exercise may be more suitable for avoiding the adverse effects of excessive high-intensity exercise on physical health.
Swimming, eccentric exercise, resistance training, and aerobic exercise are aerobic exercises. Studies have shown that aerobic exercise promotes fatty acid oxidation, improves metabolism and reduces inflammatory responses, thereby promoting M2 macrophages polarization. Anaerobic exercise may short-term increase the polarization state of M1 macrophages, thereby increasing the hepatic inflammatory response [31,32]. Therefore, aerobic exercise appears to be more beneficial than anaerobic exercise for NAFLD patients with NAFLD. In addition, resistance exercise during aerobic exercise can promote muscle growth and increase metabolic activity, promote the polarization of M2 macrophages and reducing inflammation; however, no one study has investigated its therapeutic effect on NAFLD [33,34]. Therefore, it is a better choice to choose Swimming, eccentric exercise and other sports that have been proven to improve the pathological state of NAFLD.
Both acute and continuous exercise training have been shown to induce macrophage polarization towards the M2 phenotype, and improve adipocyte and inflammatory status, as well as insulin signaling, at different durations of exercise. However, comparing the two indicates that, the effect of acute exercise is temporary and limited. Continuous training reduces fat accumulation and inflammation in the liver through a stable mechanism, and has a long-term therapeutic effect. Therefore, it is beneficial for patients with NAFLD to insist on regular long-term exercise.
In summary, although different types of exercise may affect macrophage polarization and NAFLD treatment differently, Long-term regular moderate-intensity aerobic exercise is currently the most effective therapy for NAFLD. At the same time, formulating a comprehensive treatment plan based on the patient’s physical condition and goals, combined with improvements in diet and lifestyle, is currently the most healthy and effective way to treat NAFLD.
DISCUSSION
In this study on the role of exercise-induced macrophage polarization in regulating NAFLD-targeted therapy, the primary pathological mechanism of NAFLD, the effect of the polarization of liver macrophages on NAFLD, the impact of exercise on macrophage polarization, and the mechanism and target of macrophage polarization in the treatment of NAFLD, the following conclusions ware drawn.
(1) The main factors that induce of NAFLD are a highfat diet, obesity, insulin resistance (IR), changes in the gut microbiota, and genetic variation in susceptibility.
(2) Drug treatment, nutrient induction, microfactor induction, physiological environment induction and other factors can induce hepatic macrophages polarization and affect NAFLD.
(3) Different intensities, types, and exercise frequencies affects macrophage polarization differently, and may also affect improving liver inflammation, fibrosis, and NAFLD. Currently, regular moderate-intensity aerobic exercise is the most effective therapy for treating NAFLD.
Solving NAFLD with exercise involves strategies to alter macrophage polarization by inhibiting M1 or driving M2 activation. However, research on the mechanisms of different types of exercise-mediated macrophage polarization and the differences in therapeutic effects are not yet sufficient. Future research is necessary to investigate the precise mechanisms underlying the preventive and restorative effects of different types of exercise in NAFLD, and the differences in therapeutic effects.