Anti-obesity effects of Camellia (Camellia oleifera Abel) oil treatment on high-fat diet-induced obesity in C57BL/6J mice
Article information
Abstract
[Purpose]
In the current study, we investigated the effects of camellia oil and camellia oil infused with herbs (Camellia oleifera Abel) on obesity in obese mice fed a high-fat diet (HFD).
[Methods]
The antioxidant activity of camellia oil in scavenging free radicals was investigated. Additionally, body and organ weight changes, serum and liver marker parameters, antioxidant enzyme activities, liver and epididymal fat histology, protein and gene expression associated with lipogenesis and hyperglycemia effect on adenosine monophosphate-activated protein kinase (AMPK) phosphorylation, were examined in HFD-induced obese mice.
[Results]
The hepatic steatosis and epididymal fat were significantly reduced by the oral administration of camellia oil and herb-infused camellia oil. Moreover, hepatic and serum marker parameters such as total cholesterol, insulin, triglycerides, tumor necrosis factor-α, adiponectin, thiobarbituric acid reactive substances, aspartate aminotransferase, and alanine transaminase were beneficially impacted. Additionally, the activity of antioxidant enzymes also increased. Camellia oil and herb-infused camellia oil treatments reduced the expression of genes linked to hyperglycemia and lipogenesis via activation of AMPK phosphorylation.
[Conclusion]
For many people, exercise poses an obstacle in the daily routine due to lack of ease, difficulty in maintaining consistency, and hard work. Camellia oil combined with herbs has anti-obesity and antihyperglycemic effects. These findings indicate that treatment with herb-infused camellia oil is most beneficial for elderly individuals who do not prefer frequent exercise.
INTRODUCTION
Obesity is the excessive or abnormal accumulation of body fat due to a variety of factors, including lack of exercise, excessive food intake, and heredity [1]. Obesity is the sixth most common cause of mortality [2]. Moreover, it is well established that consuming excessive fat can result in an increase in body’s adipose tissues, which leads to obesity and an increase in the risk of several diseases, including diabetes, kidney disease, fatty liver disease, atherosclerosis, hypertension, and coronary heart disease [3-5]. A safe and effective preventive method is urgently needed due to the number of affected individuals [6].
Obesity causes excessive fat accumulation in the liver because adipose tissue has a limited capacity to store fat [7] which leads to production of reactive oxygen species (ROS) [8]. ROS are believed to cause liver macrophages to switch from an anti-inflammatory to a pro-inflammatory activation state, resulting in insulin resistance [9]. Preclinical research has used C57BL/6J mice as a polygenic model for diet-induced obesity because the mechanism of obesity in these mice is comparable to that in humans [10,11]. To better understand the pathogenic mechanisms of obesity-related disorders, high-fat diets (HFD), which comprise 45–60% dietary fat, are frequently utilized in animal disease models, such as those for diabetes, liver disease, and cardiovascular diseases [12-14]. Few anti-obesity medications are used in clinical settings and have substantial side effects [15,16]. Diet with regular exercise are important factors in the prevention of obesity. Herbal products and particular foods have significant potential to actively promote weight control because of their capacity to prevent fat accumulation and reduce body weight. This is because they increase thermogenesis, fat oxidation, and energy expenditure [17]. Therefore, understanding the regulatory mechanism of obesity (abiogenesis) is crucial for its prevention and the conceptualization of anti-obesity diets.
Camellia oil is a type of edible oil with strong medicinal and nutritional qualities used in China that is made from Camellia oleifera Abel seeds [18,19]. It contains high levels of carotene, vitamin E, polyphenols, and unsaturated fatty acids such as linoleic, palmitic, and oleic acids, all of which are crucial phytochemicals for fat metabolism [20]. Previous pharmacological investigations have claimed that camellia oil has anti-obesity, anti-inflammatory, and anti-weight-gain properties [20-23]. Importantly, several studies revealed that camellia seed oil had positive effects on liver protection and hypolipidemia in HFD-fed mice [24-26]. Additionally, the Food and Agriculture Organization suggested that camellia oil is a beneficial plant oil [27]. Rosemary (Rosmarinus officinalis L.) has been used to treat various chronic illnesses since ancient times. Current studies have demonstrated the potential of rosemary in the treatment of diabetes and obesity [28]. Rosemary contains high concentrations of phenolic diterpenes, triterpenes, phenolic acids, and flavonoids, which have antioxidant, anti-hyperlipidemic, and anti-hyperglycemic properties. Numerous studies have shown that rosemary consumption offers several health advantages, such as a reduced risk of obesity, diabetes, and other metabolic syndromes [29-32]. Similarly, Syzygium aromaticum (L.), often known as cloves, contains phytochemicals such as alkaloids, tannins, flavonoids, and phenols, which are responsible for some of its biochemical characteristics. Animals treated with S. aromaticum have been shown to benefit from its anti-diabetic, anti-inflammatory, antibacterial, aphrodisiac, and antioxidant capabilities [33-35]. A recent study investigated silicone-infused camellia seed oil as an anti-icing/frosting material for food-freezing applications [36].
According to the literature survey, camellia oil, rosemary and cloves have unique antioxidant capabilities with anti-inflammatory and anti-obesity effects. However, to compare the performance of a product infused with herbs (rosemary and cloves) to that of a product containing 100% camellia oil, scientific verification of the efficacy of the product is required as well as in vitro and in vivo testing for antioxidant activity and anti-obesity properties. However, the effects of camellia oil alone and herb-infused (rosemary and cloves) camellia oil on liver and fat inflammation in HFD-induced obese mice have not yet been studied. However, the current study investigated that the effects of camellia oil (Cam) and camellia oil infused with rosemary and cloves (Cam+Herb) on oxidative stress, obesity, and morphological changes in the liver and adipose tissues of C57BL/6J mice fed with a saturated fat-rich diet.
METHODS
Preparation of camellia oil and herb-infused camellia oil, and their antioxidant activity
Camellia oil was purchased from an agricultural company, GOMARI Co., Ltd. (Yeosu-si, Jeollanam-do, Korea), and was made from camellia seeds and to prepare herb-infused camellia oil, the oil was infused with 5% rosemary and clove at a 1:1 ratio. For subsequent experimentation, camellia oil and herb-infused oil were kept in an airtight container at 4 ˚C for a maximum of one year.
Using 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals, the in vitro scavenging ability of camellia oil and herb-infused camellia oil was evaluated. We have modified the DPPH radical scavenging technique and described the DPPH radical scavenging activity analysis method [37]. Three replicate experiments were performed using 96-well plates. We prepared several quantities of camellia oil and herb-infused camellia oil (0.625, 1.25, 2.5, 5, and 10 mg/mL) along with a methanolic 0.208 mM DPPH solution. Next, 50 μL of each sample was combined with 150 μL of the DPPH radical solution. The reaction mixture containing 96-well plate was incubated at room temperature in complete darkness for 20 min before the absorbance was measured at 540 nm. The percentage (%) of DPPH radicals inhibited was determined by using the following formula: radical scavenging activity (RSA) = 100 × (Acontrol − Asample)/Acontrol, where Acontrol and Asample are absorbance values.
Animals
Male C57BL/6J mice aged five weeks (15–21 g body weight [BW]) were obtained from Orient Bio (Seongnam, Korea). The animals were housed in accordance with the Chonnam National University Guidelines for the Care and Use of Laboratory Animals. Before the study, they were acclimated for a week in a room with 55.5% humidity control and 12 h light/dark cycle.
Experimental design
This research is aimed to determine the effects of camellia oil and herb-infused camellia oil treatment. A dose of 5 mL/kg BW/day was administered for a period of seven weeks, following previously published studies18-26. In this study, after one week of acclimatization, the C57BL/6J mice were randomly classified into four groups: (1) HFD-induced obese group (CON); (2) standard diet-fed normal group (NOR); (3) HFD-induced obese group treated with camellia oil (Cam); and (4) HFD-induced obese group treated with camellia oil infused with herbs (Cam+Herb). Biochemical analyses of the CON and NOR group were compared. Moreover, the beneficial effects of camellia oil (Cam group) and camellia oil infused with herbs (Cam+Herb group) were analyzed and compared with the corresponding parameters in the CON group. Further, normal (NOR) mice were provided with feed that contained the following components per kg of food: t-butyl hydroquinone (0.01 g), vitamin mix (10 g), choline bitartrate (2.5 g), and mineral mix (35 g). The CON, Cam (orally treated Camellia oil with 5 mL/kg BW/day; Daily intake ratio: 0.52), and Cam+Herb (orally treated with Camellia oil infused with herbs at 5 mL/kg BW/day; daily intake ratio: 0.53) groups were received HFD comprising the following components per kg of diet: cysteine (176.8 g), sucrose (176.8 g), casein lactic acid (200 g), malt dextrin (100 g), corn starch (72.8 g), soybean oil (25 g), cellulose (50 g), animal fat (177.5 g), minerals (50 g), choline bitartrate (2 g), vitamin mix (1 g), and dye (0.05 g). During the experimental period, the daily dietary intake and weight changes were recorded between 08:00–09:00 hours. The food efficiency ratio (FER) was estimated by calculating the body weight gain (g/day) / the daily food intake (g/day) ×100, throughout the experiment.
After seven weeks, isoflurane (Hana Pharm Co., Ltd., Seongnamm, Korea) was used to anesthetize the mice through inhalation. Serum samples were obtained by collecting blood samples in heparin tubes and centrifuging them at 2,000 × g for 10 min at 4 ˚C to determine biochemical characteristics. The organs were collected, washed with physiological saline, dried, and weighed. They were rapidly frozen using liquid nitrogen and kept for examination at -80 ˚C. The weights of fat were obtained from various locations. Hepatic and epididymal fat tissues were preserved in 10% formalin for histological analysis. The liver tissues were homogenized at a ratio of 1:9 in 450 mM phosphate buffer (pH 7) and centrifuged at 25,000 rpm at 4 ˚C for 20 min. Then the supernatant was collected to analyze lipid and antioxidant activities. The experimental methods utilized in this study were approved by the Chonnam National University’s animal ethics committee (CNU IACUC-YS-2020-9).
Serum biochemical analysis and liver total cholesterol and triglyceride levels
An ELISA kit (Elabscience Inc., USA) was used to quantify insulin (INS), thiobarbituric acid reactive substances (TBARS), tumor necrosis factor-α (TNF-α), and adiponectin [38]. Enzymatic analysis kits (Asan Pharmaceuticals, Hwasung, Korea) were used to evaluate the levels of TC, high-density lipoproteins (HDL) cholesterol, low-density lipoproteins (LDL) cholesterol, and triglycerides (TG), as well as the activities of alanine transaminase (ALT) and aspartate transaminase (AST) [39]. Total protein (Biuret test kit, Elabscience Inc., USA), blood urine nitrogen (BUN) (urease GLDH test kit, Elabscience Inc., USA), and albumin (BCG kit, Elabscience Inc., USA) were measured [40].
The activity of antioxidant enzymes in the liver tissues
The manufacturer’s instructions were followed to determine glutathione (GSH), glutathione reductase (GR), glutathione peroxidase (GPX), superoxide dismutase (SOD) and glutathione S-transferases (GST) activities in the liver tissue using a colorimetric test kit (Biovision Inc, San Francisco, CA, USA) [41].
Staining with hematoxylin and eosin (H&E) for histological examination
Tissues were initially fixed with formalin (10% [v/v]) in phosphate buffer before being embedded in paraffin wax for histological investigation. Subsequently, H&E stain was applied to each of the 4–6 μm thick portions. A camera and optical microscope (Olympus DP70, Olympus Optical Co., Tokyo, Japan) were used to observe histological alterations [42].
Total protein extracts from liver tissue and western blotting
With a few minor modifications, the procedures described in [43] were used to isolate proteins from the liver for western blot analysis. Briefly, a solution containing 0.1% sodium dodecyl sulfate, 50 mM Tris, 150 mM sodium chloride, 1% sodium deoxycholate, 1% Triton X-100, 1 mM sodium orthovanadate, and 1 mM ethylenediaminetetraacetic acid was used to homogenize liver tissues (Polytron CH-6010; Kinematica GmbH, Luzern, Switzerland). The homogenate was collected and centrifuged at 12,000 rpm at 4 ˚C for 20 min (MX-160 from Tomy Seiko Co., Ltd.). Total cell lysate was preserved in the final supernatant. Then, as previously mentioned [44], western blotting for 5’ adenosine monophosphate-activated protein kinase (AMPK) using primary antibody (Cell Signaling, cat #2532, Danvers, MA), phosphorylated form of α-AMPK (pAMPK) using primary antibody (Cell Signaling, cat #2535, Danvers, MA), Fatty acid synthase (FAS) using primary antibody (Cell Signaling, cat #3180, Danvers, MA), acetyl-CoA carboxylase (ACC) using primary antibody (Cell Signaling, cat #3662, Danvers, MA), and the HRP-linked secondary antibody (Cell Signaling, cat #7074, Danvers, MA) was performed with liver homogenate containing 100 μg protein.
RNA isolation and quantification using real-time RT-PCR
Utilizing the TRIzol RNA isolation reagent (Invitrogen, Carlsbad, CA, USA), total RNA was extracted from liver tissue. According with the manufacturer’s instructions RNA reverse transcription was carried out using the Prime ScriptTM 1st Strand cDNA Synthesis Kit (Takara Bio Inc., Shiga, Japan). Twenty microliters of SYBR® Premix Ex Taq (Takara Bio Inc.) was used for quantitative RT-PCR. The results were adjusted for mRNA signals against β-actin. The primer sequences in Table 1 was used in this study [45].
Statistical analysis
The means ± standard deviation (SD) of the data are displayed. To perform the statistical analyses using IBM SPSS statistic version 20 (Statistical Package for Social, SPSS Inc., Chicago IL, USA). One-way analysis of variance (ANOVA) was used to assess the data, and Tukey’s multiple comparison test was performed. Differences were deemed statistically significant at p < 0.05.
RESULTS
Antioxidant activity of camellia oil and herb-infused camellia oil
DPPH radical scavenging analyses were used to assess the antioxidant activities of camellia oil and herb-infused camellia oil, as shown in Figure 1. The DPPH radical scavenging activity was considerably enhanced in a concentration-dependent manner (from 0.625 mg/mL to 10 mg/mL). Moreover, IC50 value (20.05 mg/mL) of herb-infused camellia oil was less than that of pure camellia oil IC50 value (55.17 mg/mL), indicating that the herb-infused oil has a greater scavenging ability than that of camellia oil alone.
Metabolic characteristics of camellia oil and herb-infused camellia oil treated HFD-fed mice
We analyzed the in vivo effects of camellia oil and herb-infused camellia oil, the FER, BW, organ weight, and total fat content were analyzed (Table 2). In contrast to the CON group, food consumption, BW gain, and FER were significantly lower in the Cam and Cam+Herb groups after 7 weeks of treatment. Moreover, the kidney, heart, and liver weights were significantly lower in Cam and Cam+Herb groups than in the CON group. Whereas, gastrocnemius muscle weight was not significantly different. Fat content analysis indicated that mesenteric fat, retroperitoneal, epididymal, kidney, and total fat contents were significantly higher in the HFD-induced CON group than in the Cam and Cam+Herb groups.
Effect of camellia oil and herb-infused camellia oil on hepatic and serum marker enzymes including anti-hyperlipidemic effect in HFD-induced obese mice
We analyzed the hepatic and serum markers associated with obesity in mice treated with camellia oil and herb-infused camellia oil. Compared to those of the HFD fed CON group, the Cam and Cam+Herb groups had significantly lower hepatic TC and TG levels, as well as serum TC and TG levels (Table 3). To establish the anti-obesity activity of camellia, we examined blood markers linked to obesity. Compared with those of the NOR, the CON group had higher levels of the marker enzymes AST, ALT, insulin, TNF-α, BUN, LDL cholesterol, and TBARS. However, this increase was dramatically reduced in the Cam and Cam+Herb groups. The adiponectin and HDL cholesterol levels are high in Cam group than in the CON group. However, there were no appreciable differences in blood protein and albumin levels between the four groups.
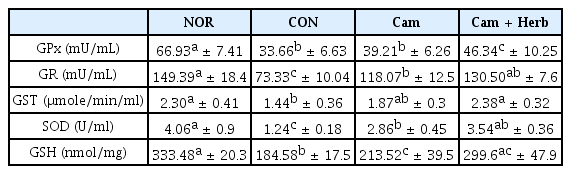
Hepatic antioxidant enzyme activities in camellia oil and herb-infused camellia oil treated HFD-induced obese mice
GPx, GR, GST, and SOD activities and GSH levels were assessed in hepatic homogenates to determine the ability of camellia oil and herb-infused camellia oil to prevent liver impairment in HFD-induced obese mice. As shown in Table 4, the CON group had lower hepatic GSH levels and lower GPx, GR, GST, and SOD enzyme activities than those of the NOR group. GSH levels and antioxidant enzyme activities were both increased by camellia oil treatment after 7 weeks (Cam, Cam+Herb). Moreover, the enzyme activities of the Cam+Herb group were higher than those of the CON and Cam groups.
Histological changes of hepatic and epididymal fat in camellia oil and herb-infused camellia oil treated HFD fed mice
The histological changes induced by camellia oil and herb-infused camellia oil on the liver and epididymal fat are shown in Figure 2. The mice in the CON group of the HFDfed animals had more severe macrovesicular steatosis than the mice in the NOR group, according to the liver histology findings using H&E staining, as shown in Figure 2(A). Additionally, the liver weight of the CON group increased with strong fat (+++) deposition owing to a significant buildup of hepatic lipid droplets. Moreover, HFD-fed CON group also had a high FER, which contributed to obesity (Table 1). HFD-fed mice are known to grow heavier than mice fed conventional diets are. However, both camellia oil and herb-infused camellia oil (Cam, Cam+Herb) treatments reduced macrovesicular steatosis. Moreover, the deposition of fatty acids decreased to mild (+); in addition, the liver weight decreased significantly compared with the CON group.

Effect of camellia oil and herb-infused camellia oil on the histology of mouse tissues (A) liver fat contents, and (B) epididymal fat adipocyte area in NOR, CON, Cam, and Cam+Herb groups.
Examination of the histological changes in the epididymal tissues showed that the adipocytes of the HFD-fed CON obese group were significantly larger than those of the NOR group which was fed a normal diet [Figure 2(B)]. However, compared to those of the CON group, the adipocytes in the camellia groups (Cam and Cam+Herb) were significantly smaller. Additionally, the total adipocyte area of the CON group was greater (547 μm2) than that of the NOR group (320 μm2). The overall adipocyte area dramatically decreased in both the Cam group (480 μm2) and the Cam+Herb group (426 μm2). These findings demonstrate that treatment with camellia oil and herb-infused camellia oil reduces intracellular lipid accumulation in adipocytes.
Effects of camellia oil and herb-infused camellia oil treatment on AMPK, p-AMPK, FAS, and ACC protein expressions, in high fat diet-induced obese mice
Using camellia oil and herb-infused camellia oil, we examined the AMPK and p-AMPK protein expression in HFD-induced obese mice. As shown in Figure 3(A–C), the CON group mice showed that the hepatic AMPK and p-AMPK expression was downregulated. However, these levels dramatically increased in post-treatment with the camellia oil and herbs infused with camellia oil groups. In addition, compared to that in the CON group, the ratio of pAMPK/AMPK levels increased in the Cam and Cam+Herb groups. These findings provide indirect evidence that camellia oil and herb-infused camellia oil exert anti-obesity effects by increasing the levels of AMPK and p-AMPK protein expression in HFD-induced mice.

Effect of camellia oil and herb-infused camellia oil on the (A) expression of AMPK and p-AMPK in liver tissue is analyzed by western blotting using antibodies against AMPK and p-AMPK, (B) Protein bands are quantified by ImageJ. β-actin is used as control to evaluate relative expression of protein, (C) pAMPK/AMPK ratio. Each statistic is the mean ± standard deviation (SD) (n=4). Values that do not share a common letter are substantially different at p < 0.05 when applying Tukey’s multiple comparison test.
Additionally, we examined the expression of FAS and ACC in the NOR, CON, and camellia groups (Cam and Cam+Herb). As shown in Figure 4(A, B), the livers of the HFD-induced CON group mice showed higher expression levels of ACC and FAS proteins. Whereas, camellia and herb-infused camellia oil treatment reduced ACC and FAS levels, the herb-infused group more effectively lowered protein expression. These findings suggest that herb-infused camellia oil has a stronger anti-obesity effect than that of camellia oil alone by reducing the production of fatty acids in mice on HFD.

Effect of camellia oil and herb-infused camellia oil on the (A) expression of FAS and ACC in liver tissue are analyzed by western blot, (B) Protein bands are quantified by ImageJ. β-actin is used as control to evaluate relative expression of protein. Each statistic is the mean ± standard deviation (SD) (n=4). Values that do not share a common letter are substantially different at p < 0.05 when applying Tukey’s multiple comparison test.
Effects of camellia oil and herb-induced camellia oil on hepatic mRNA levels associated with lipogenesis and gluconeogenesis in HFD-induced obese mice
The mRNAs expression in liver is linked to lipogenesis and gluconeogenesis was assessed in HFD-induced obese mice (CON) and camellia oil-treated groups (Cam, Cam+Herb). These findings demonstrate that phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) enzyme expression levels were increased in the CON group, which is consistent with an increase in hepatic glycogen and blood glucose levels. PEPCK and G6Pase RNA expressions was considerably inhibited (p < 0.05) during the 7-week treatment with camellia oil and herb-infused camellia oil, as shown in Figure 5(A, B). However, liver glycogen phosphorylase (LGP) expression increased and glycogen synthase (GS) expression decreased in the HFD-fed CON group, indicating that liver glycogen synthesis was reduced. This further demonstrates the link between fat and insulin resistance, which favors the onset of diabetes. However, after receiving a 7-week treatment with camellia oil and herb-infused camellia oil, GS levels considerably increased, whereas LPG levels declined, as shown in Figure 5(C, D). Additionally, the expression of the lipogenic enzymes ACC, FAS, and stearoyl coenzyme A desaturase (SCD) was enhanced in the CON group, although the hepatic mRNA expression levels in the camellia oil-treated groups (Cam and Cam+Herb) were significantly reduced (Figure 5(E-G). Compared to those in the NOR, the CON group had lower levels of carnitine palmitoyltransferase (CPT) gene expression, which is associated with fatty acid oxidation. Figure 5(H) shows that the decline in CPT expression levels was substantially higher in the groups that received camellia treatment. Additionally, Figure 5(I) shows that pyruvate dehydrogenase kinase (PDK) gene expression was increased in the CON group, whereas it was significantly reduced in the camellia oil-treated groups (Cam, Cam+Herb).

Effect of camellia oil and herb-infused camellia oil on the mRNA expression of glucose and lipid metabolism related genes. (A) phosphoenolpyruvate carboxykinase (PEPCK), (B) glucose-6-phosphatase (G6Pase), (C) glycogen synthase (GS), (D) liver glycogen phosphorylase (LGP), (E) fatty acid synthase (FAS), (F) acetyl-CoA carboxylase (ACC), (G) stearoyl coenzyme A desaturase (SCD), (H) pyruvate dehydrogenase kinase (PDK) and fatty acid oxidation related genes (I) carnitine palmitoyltransferase (CPT) in the liver. Each statistic is the mean ± standard deviation (SD) (n=6). Values that do not share a common letter are substantially different at p < 0.05 when applying Tukey’s multiple comparison test.
DISCUSSION
Camellia oil, an edible oil with very high nutritional value, is rich in unsaturated fatty acids and phytochemicals such as squalene, sterols, tocopherols, and polyphenols [46]. These compounds also possess a variety of bioactivities, including antioxidant, anticancer, and anti-inflammatory properties [47]. Moreover, herbs (rosemary and cloves) are rich in phytochemicals, such as alkaloids, tannins, flavonoids, and phenols, which are responsible for some of their biochemical characteristics. Owing to its economic advantages, camellia oil is universally recognized as a natural source of antioxidants. It contains several organic compounds and exhibits improved radical-scavenging performance in the DPPH and ABTS (2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)) assays [48]. In this study, camellia oil (Cam) and herb-infused camellia oil (Cam+Herb) showed lower IC50 values, indicating a stronger capacity to scavenge DPPH radicals. Additionally, compared to those observed in the camellia oil-treated group, the herb-infused camellia oil treatment had a stronger scavenging ability and lower IC50 value. The antioxidant properties of the phenolic compounds tannins, flavan-3-ols, catechol, and pyrogallol in camellia oil were assessed by Gao et al. [49], who concluded that they have considerable DPPH radical scavenging activity. According to previous reports, the scavenging of hydroxyl radicals by flavanols and catechins, including camellia oil, have shown that the amount of hydroxyl radicals affects how organic matter reacts [50]. Further, the present experimental findings show that the pretreatment of cells with camellia oil can lower ROS generation.
In numerous strains of mice and rats, high-fat foods have been shown to increase body weight and cause diabetes. C57BL/6J mice, which develop obesity, hyperinsulinemia, hyperglycemia, and hypertension when fed a HFD ad libitum, are a particularly effective model for simulating human metabolic abnormalities observed in obesity [51]. This is primarily caused by an increase in the consumption of foods that are high in calories and have high levels of sugar and saturated fats (HFD), along with a decrease in physical activity. The increase in size and quantity of adipocytes in adipose tissues are considered to have a cellular syndrome of obesity, and the increase in adipocyte size is due to increased lipid cellular storage [52]. According to Avtanski et al. [53], male C57BL/6 mice are characterized by excess body weight, fat deposits, liver accumulation, and inflammation after receiving HFD for 9 weeks. The current study, we examined the anti-obesity effects of camellia oil and herb-infused camellia oil in HFD-fed mice. Camellia treatment reduced body weight gain by lowering the mass of the liver and visceral white adipose tissue and marginally increasing insulin sensitivity in HFD-fed mice. Moreover, our data revealed that the HFD-fed mice had higher hepatic TC and TG levels. This indicated that the HFD diet led to excess fat accumulation in the liver tissue compared to that in the camellia-treated groups. It indicated that camellia treatment has a beneficial effect in reducing aberrant fat accumulation in the hepatic tissue. The liver weight of the HFD-induced CON group was not substantially different from that of the NOR group because HFD-induced mice may eat less as their appetite is reduced [54]; however, the hepatic TC and TG levels were significantly higher than those of the normal (NOR) and camellia-treated groups (Cam and Cam+Herbs) due to excessive fat accumulation in HFD-induced mice. Nagao et al. [55] investigated how LDL cholesterol, body weight, and fat mass, especially the visceral fat region, could be reduced in humans by treatment with catechin-containing camellia oil. Li et al. [56] investigated the effects of camellia and found that the plant’s primary chemical constituents had dose-dependent anti-adipogenic activity and significantly reduced the accumulation of lipid droplets in liver and fat tissues.
Histological analysis of the liver tissues of CON group revealed vacuole development with fat accumulation, hepatocellular ballooning, and microvesicular steatosis. In mice belonging to the CON group, fatty liver was a result of increased levels of metabolic indicators, such as TC, AST, ALT, and TG [57]. In addition, HFD-induced control obese mice (CON group) also had higher body weights than those of normal mice, along with an adipose tissue weight increase caused by deposition of fat including larger adipocytes. Moreover, the blood levels of adiponectin, TNF-α, TBARS, and LDL cholesterol are thought to be the main contributors to chronic inflammation linked to obesity [58-62]. Camellia oil and herb-infused camellia oil gradually decreased inflammatory vesicular steatosis. In addition, the anti-obesity effect of camellia oil decreased the serum levels of adiponectin, TNF-α, TBARS, and LDL cholesterol. Further, the LDL cholesterol level in the Cam+Herb group decreased more significantly than that in the Cam group. These findings imply that herb-infused camellia oil has more anti-obesity effects than those of camellia oil alone in the context of the fatty liver.
Obese mice in the HFD-induced control group (CON) have a pro-oxidant condition due to their visceral adipose tissue and liver can generates the pro-inflammatory cytokines [63] and have lower antioxidant enzyme activity [64]. According to the results of the present study, HFD-induced obesity in CON mice led to increased oxidative stress and inflammation in the liver, which in turn decreased the activity of antioxidant enzymes such GPx, GR, GST, and SOD as well as GSH levels. This decline in antioxidant enzyme activity may be caused by the rapid and exhausting use of antioxidant enzymes that have been stockpiled to fight free radical production in obesity; obesity-associated oxidative stress-involves processes that can enhance fat deposition in organs and free radical production [65]. In addition, high levels of lipid peroxidation, hypertension, and hypercholesterolemia are plausible causes of elevated oxidative stress in obesity [66]. The antioxidant enzyme activities increased in HFDfed mice upon treatment with camellia oil and herbs-infused camellia oil. Additionally, herbs-infused oil treatment more efficiently boosts antioxidant activities than the effects of camellia oil treatment [67]. The reduction of hepatic antioxidant enzyme activity was successfully restored by herbs-infused camellia treatment in HFD-induced obese CON mice, indicating that herb-infused camellia oil treatment is advantageous for increasing antioxidant effects in obese mice.
Owing to the rising prevalence of pathogenic metabolic diseases, AMPK activity is dysregulated in obese mice [68]. Obesity and high insulin resistance correlate with reduced AMPK activation [69,70]. Our findings revealed that the HFD-induced obese CON group exhibited lower AMPK and p-AMPK protein expression. According to the dysregulation of AMPK signaling or the absence of AMPK expression in obese mice, lower AMPK activity is associated with the etiology of obesity [71]. In HFD-induced obese mice, camellia oil and herb-infused camellia oil treatment activated AMPK and phosphorylated AMPK (p-AMPK) and decreased ACC, thereby promoting fatty acid oxidation [72]. p-AMPK is an essential protein that controls lipid and carbohydrate metabolism [73].
An important enzyme for the production of long-chain fatty acids is ACC, along with FAS, it can control the pace of hepatic triglyceride synthesis. According to the current findings, obese CON group that were fed an HFD had higher levels of FAS and ACC than those of the NOR group. As a result, it plays a significant role in the onset of fatty liver [74]. Elevated hepatic FAS and ACC levels are mostly caused by high insulin levels [75]. Additionally, the HFD-fed CON group displayed elevated FAS and ACC expression without promoting liver phosphorylation. Following treatment with camellia oil and herb-infused camellia oil, the increased FAS and ACC levels were reduced. According to these findings, treatment with herb-infused camellia oil lowered the levels of FAS and ACC and liver steatosis by activating AMPK and p-AMPK.
Increased expression of lipogenic enzymes (FAS, ACC, and SCD), gluconeogenic enzymes (PEPCK and G6Pase), and the AMPK protein expression may assist to explain the elevated liver weight, including hepatic steatosis, in HFD-induced obese CON mice. The hepatic gluconeogenic enzymes expressions can cause chronic hepatic glucose production, hyperglycemia, and insulin resistance in obese and diabetic animals [76]. Our findings showed that PEPCK and G6Pase mRNA levels increased in the HFD-induced obese CON group, indicating that the HFD-fed mice produced excess glucose. To maintain glucose homeostasis, these levels were reduced in the liver tissues of the camellia oil (Cam) and herb-infused camellia oil (Cam+Herb) groups.
The activities of GS and LGP regulate the ability of the liver to synthesize glycogen. In the context of lipid accumulation, glycogen production is reduced concurrently with declining expression of GS [77]. These findings suggest that lowering AMPK levels, fat storage, and hepatic steatosis occurred in obese mice (CON) by increasing the expression of mRNAs encoding the lipogenic enzymes ACC, FAS, and SCD. The use of camellia oil and herb-infused camellia oil may stimulate phosphorylated AMPK signaling and prevent the mRNA expression of the transcription factors ACC, FAS, and SCD, resulting in decreased mRNA transcription and downregulation of enzymatic activity [78,79]. This suggests that camellia and herb-infused camellia oils inhibit lipogenesis and successfully prevent hepatic steatosis in HFD-induced obese mice. When the mRNA expression of CPT is suppressed, there is a deficit in fatty acid oxidation, which results in fatty liver [80]. In contrast, obese mice show higher levels of PDK mRNA expression [81]. However, treatment with camellia and herb-infused camellia oils improved CPT mRNA expression and protected against fat accumulation in liver tissues.
In conclusion, our findings showed that camellia and herb-infused camellia oil have the strongest capacity to scavenge free radicals. Through the AMPK pathway in the liver and adipocytes, these positive effects may be modulated by the lipid profile and a decrease in the expression levels of lipogenic enzymes such as FAS and ACC. In addition to reducing body weight, liver steatosis, and the formation of TG and lipids in the liver and adipose tissues, the herb-infused camellia oil treatment provided a stronger anti-obesity effect than that of camellia oil treatment. This suggests that treatment with the herb-infused camellia oil has a positive effect on diabetes-induced obesity in rats.
Acknowledgements
This research was supported by the Starting Growth Technological R&D Program funded by the Ministry of SMEs and Startups (MSS, Korea) (S3308965).