Effects of partial sleep deprivation after prolonged exercise on metabolic responses and exercise performance on the following day
Article information
Abstract
[Purpose]
We determined the effect of partial sleep deprivation (PSD) after an exercise session on exercise performance on the following morning.
[Methods]
Eleven male athletes performed either a normal sleep trial (CON) or a PSD trial. On the first day (day 1), all subjects performed an exercise session consisting of 90 min of running (at 75%
[Results]
On day 2, neither the MVC nor
[Conclusion]
A single night of PSD after an exercise session significantly decreased endurance performance without significantly changing muscle strength or cardiopulmonary response.
INTRODUCTION
Athletes are required to perform strenuous exercise on consecutive days to improve their performance. Therefore, it is essential for them to promote recovery of physical function (e.g., maximal strength and power output) during the post-exercise period to increase the quality of their next training session. Among the various “post-exercise treatments” that have been established, including massage, cryotherapy, nutrient intake, and sleep [1-3], sleep stands out as it is indispensable for life, physiological growth, and repair [3]. In general, 9–10 h of sleep per night is recommended to facilitate appropriate recovery following an exercise session [4]. However, a survey of 890 elite athletes revealed that 41% of the athletes experienced sleep problems (e.g., falling asleep at night) and 11% slept less than 6 h per night [5]. Furthermore, the onset of sleep among athletes can be delayed due to stress derived either from competition, daily strenuous exercise, or training and travel occurring late in the evening [6-8]. According to Oda and Shirakawa [8], high-intensity exercise before bedtime delayed sleep onset relative to moderate-intensity exercise.
Total sleep deprivation (TSD) and partial sleep deprivation (PSD) have been shown to impair exercise performance [9-13]. Oliver et al. [12] showed that a single night of sleep deprivation decreased performance during an endurance running test on the following day. Moreover, maximal aerobic power decreased by 50% after PSD [11], while heart rate and minute ventilation during submaximal exercise increased [10]. Peak oxygen consumption during exercise also decreased significantly, although the maximal workload at exhaustion was not affected. In addition to impairing endurance performance, PSD may also reduce anaerobic performance. It was found that 4 h of PSD significantly reduced both peak and mean power outputs during a 30 s maximal sprint [9]. Furthermore, PSD augmented exercise-induced elevations in plasma interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) following repeated sprint training [14], and a single night of PSD increased the transcription of IL-6 and TNF-α [15]. However, these experiments were designed to determine the impact of reduced sleep duration itself, without exercise. Considering that athletes perform consecutive days of high-intensity exercise, it is necessary to determine the impact of PSD after an exercise session. Therefore, the present study examined the effect of PSD after exercise on metabolic responses and exercise performance on the following morning. We hypothesized that a single night of PSD after exercise would reduce exercise performance when compared with a normal sleep duration.
METHODS
Subjects
Eleven male athletes participated in the study. The mean ± standard error (SE) age, height, and body mass of the study subjects were 20.8 ± 0.8 years, 168.9 ± 6.9 cm, 67.4 ± 9.8 kg, respectively. They all followed a regular sleep cycle, with an average sleep duration of approximately 6 h and 7 min. Prior to the study, all subjects were informed about the experimental procedures and possible risks involved in this study, and they subsequently provided informed consent. The present study was approved by the Ethics Committee for Human Experiments at Ritsumeikan University, Shiga, Japan.
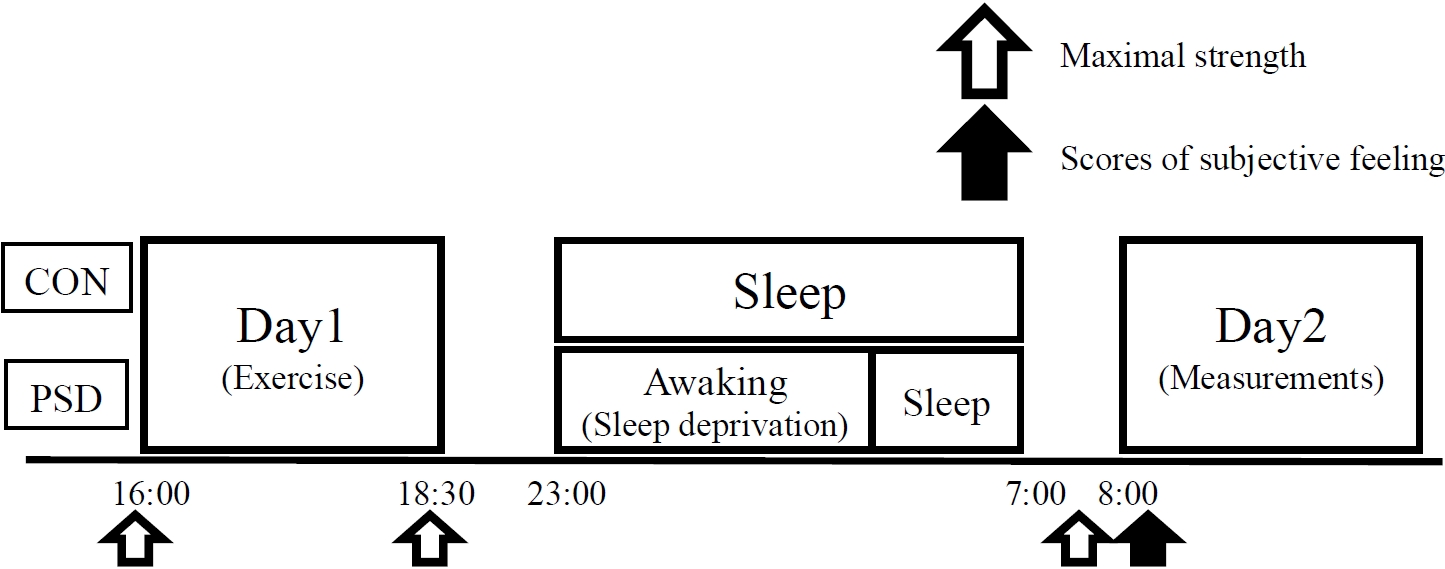
Experimental design
Habitual sleep duration was monitored for 7 days before the experiment. Sleep duration was recorded using an accelerometer (ActiGraph; Ambulatory Monitoring, Inc., Ardsley, NY, USA), and the individual sleep duration and magnitude of sleep deprivation were determined. Prior to the experiment, an incremental running test was performed to evaluate the maximal oxygen uptake (
Exercise protocol
On day 1, the exercise protocol was started with a 5 min warm-up consisting of running at 60%
Measurements
V ˙ O 2 m a x
The running velocity was initially set at 6 km/h, and then was increased by 2 km/h every 4 min until it reached 10 km/h. Once the running velocity reached 10 km/h, it was increased by 2 km/h every 3 min until it reached 14 km/h. Once the running velocity reached 14 km/h, it was increased by 0.6 km/h every minute until volitional exhaustion. During the test, expired gases were collected and analyzed with an automatic gas analyzer (AE300S; Minato Medical Science Co., Ltd., Tokyo, Japan). The collected data were averaged every 30 s. Heart rate (HR) was measured continuously during the test with a wireless HR monitor (Acculex Plus; Polar Electro Oy, Kempele, Finland).
Maximal voluntary isometric contraction of knee extension
The MVC with the knee positioned at 70° of extension (the fully extended position was defined as 0°) was measured using an isokinetic dynamometer (Biodex System 4; SAKAI Medical Co., Tokyo, Japan). The subjects exerted their maximal strength (3 s) knee extension twice, and the highest value was included in the analysis. A 60 s rest period was provided between extensions.
Subjective assessments
Subjective fatigue, leg muscle soreness, sleepiness, quality of sleep, and falling asleep were evaluated using a 100 mm visual analog scale. The subjects provided ratings for respiratory strain (RPE-R) and leg strain (RPE-L) every 15 min during the running sessions on days 1 and 2 using a 10-point scale of perceived exertion [16].
Physiological responses
On day 2, oxygen uptake (
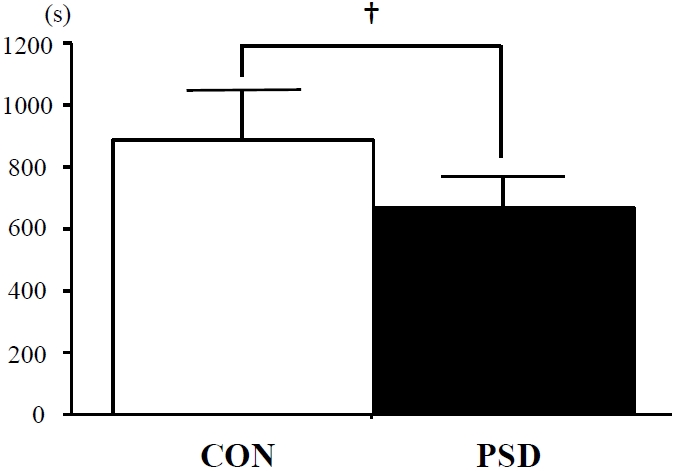
Time to exhaustion
An all-out running test at 85%
Statistical analysis
All data are expressed as the mean ± SE. Two-way analysis of variance (ANOVA) with repeated measures was used to confirm the interaction (trial × time) and main effects (trial and time). When the ANOVA revealed a significant interaction or main effect, the Tukey-Kramer test was performed as a post-hoc analysis to identify differences. For all tests, a p-value less than 0.05 was considered statistically significant.
RESULTS
Subjective variables
Subjective scores of fatigue, leg muscle soreness, sleepiness, and sleep quality were evaluated on day 2. The sleepiness score was significantly higher in the PSD trial than in the CON trial (p < 0.001), whereas the vitality score was significantly lower in the PSD trial than in the CON trial (p < 0.05). The scores of subjective fatigue, muscle soreness, and sleep were not significantly different between the two trials.
Cardiorespiratory variables and time to exhaustion during the submaximal running test
Figure 2 shows the change in respiratory variables during the 20 min of submaximal running on day 2. The results show that
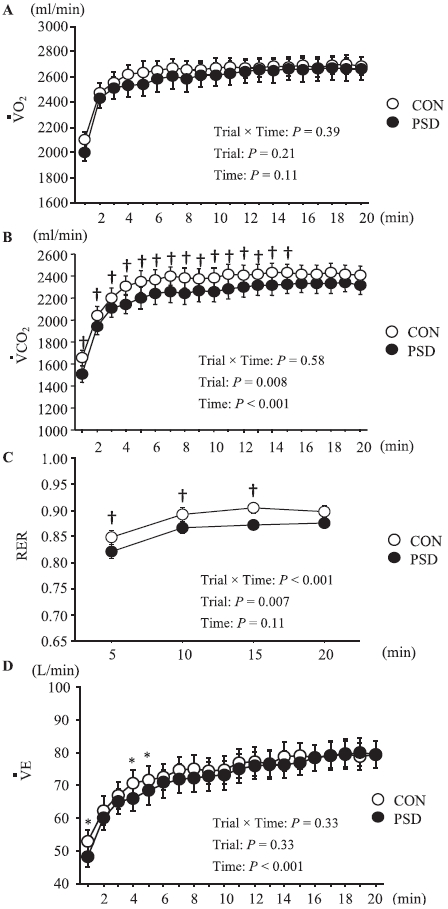
Oxygen uptake (
The TTE during running at 85%
Maximal voluntary isometric contraction (MVC) of knee extension
The changes in the MVC of knee extension are shown in Table 1. In the PSD trial, a significant decrease in MVC values was observed on day 2 when compared to day 1 (main effect for time, p < 0.05), whereas no significant change was observed in the CON trial. However, no significant difference between the PSD and CON trials was observed at any time point.
DISCUSSION
The present study investigated the effect of PSD after a prolonged exercise session on exercise performance on the following morning. The results showed that neither the
The RER during 20 min of submaximal running at 75%
Despite a marked reduction in endurance performance in the PSD trial, the influence of PSD on MVC was small, and there was no significant difference in MVC between the CON and PSD trials. In a previous study, sleep restriction after strenuous exercise did not attenuate peak isokinetic torque on the following morning [19]. Bambaeichi et al. [27] demonstrated that PSD (2.5 h of sleep) did not significantly alter maximal strength or core temperature in the morning (6:00) or evening (18:00). Moreover, 60 h of sleep deprivation did not attenuate MVC compared to that under normal sleep conditions [28]. These findings are consistent with the results of our PSD trial.
Some limitations must be carefully considered when interpreting these results. First, we were not able to evaluate muscle glycogen content, which might explain the lower endurance performance in the PSD trial. Second, we measured the effect of PSD after an exercise session only on the following morning, thus it is unclear how long the impairment of endurance performance lasts. Third, although we focused on the impact of reduced sleep duration, sleep quality is also important for promoting recovery following exercise. Therefore, it would be meaningful to evaluate sleep quality after exercise in future studies. Finally, the present PSD trial deprived the subjects of sleep during the first part of the night. However, in some previous studies, sleep was deprived during the latter part of the night [9, 19, 26]. Studies to determine the impact of sleep deprivation in different phases of the night would be interesting.
In conclusion, a single night of PSD after exercise did not markedly affect the strength or
Acknowledgements
We would like to thank all the subjects who participated in the study. No specific funding was received in support of this study.