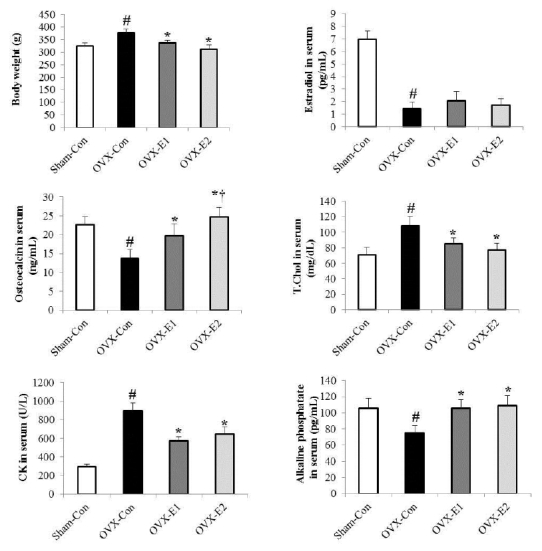
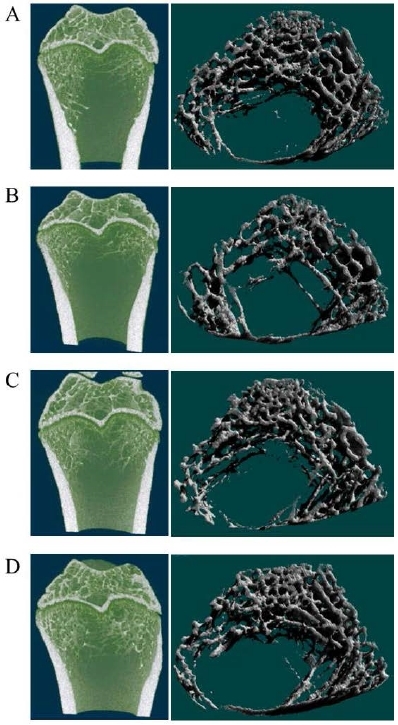
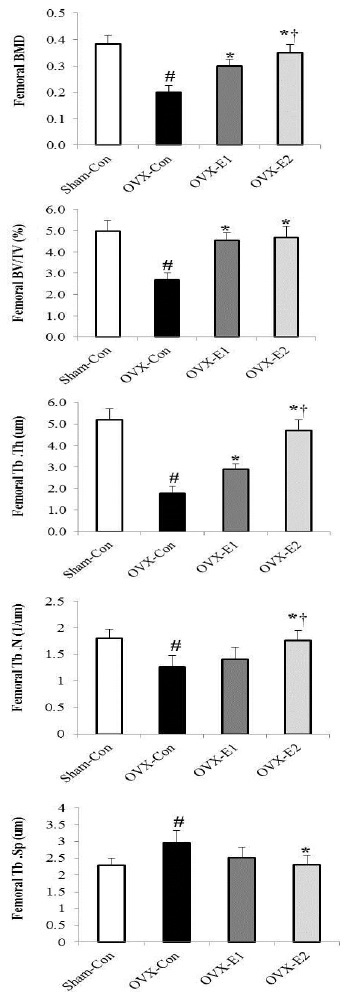
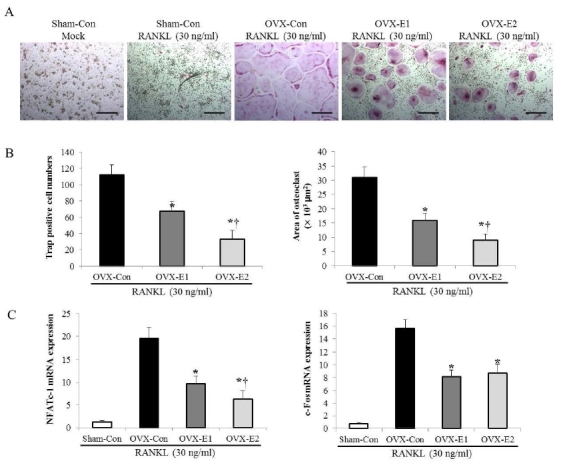
1.
Golob AL, Laya MB. Osteoporosis: screening, prevention, and management. Med Clin North Am. 2015; 99: 587-606.

Golob AL, Laya MB. Osteoporosis: screening, prevention, and management.
Med Clin North Am 2015;99:587-606. PMID:
25841602.
2.
Kannus P, Parkkari J, Niemi S, Pasanen M, Palvanen M, J├żrvinen M, Vuori I. 2000. Prevention of hip fracture in elderly people with use of a hip protector. N Engl J Med. 2000; 343: 1506-13.

Kannus P, Parkkari J, Niemi S, Pasanen M, Palvanen M, J├żrvinen M, Vuori I. 2000. Prevention of hip fracture in elderly people with use of a hip protector.
N Engl J Med 2000;343:1506-13. PMID:
11087879.
4.
Liu G, Peacock M. Age-related changes in serum undercarboxylated osteocalcin and its relationships with bone density, bone quality, and hip fracture. Calcif Tissue Int. 1998; 62: 286-289.


Liu G, Peacock M. Age-related changes in serum undercarboxylated osteocalcin and its relationships with bone density, bone quality, and hip fracture.
Calcif Tissue Int 1998;62:286-289. PMID:
10.1007/s002239900432. PMID:
9504950.
5.
Currie JL, Harrison MB, Trugman JM, Bennett JP, Wooten GF. Postmenopausal estrogen use affects risk for Parkinson disease. Archives of Neu Rology. 2004; 6: 886-888.

Currie JL, Harrison MB, Trugman JM, Bennett JP, Wooten GF. Postmenopausal estrogen use affects risk for Parkinson disease.
Archives of Neu Rology 2004;6:886-888.
6.
Korkia P. Osteoporosis: process, prevention, and treatment. J Bodyw Mov Ther. 2002; 6: 156-169.

Korkia P. Osteoporosis: process, prevention, and treatment.
J Bodyw Mov Ther 2002;6:156-169. PMID:
10.1054/jbmt.2001.0273.
7.
Gambacciani M, Pepe A. Menopause and related problems. Minerva Med. 2007; 98: 191-201.
Gambacciani M, Pepe A. Menopause and related problems.
Minerva Med 2007;98:191-201. PMID:
17592440.
8.
Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov. 2012; 11: 234-250.


Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention.
Nat Rev Drug Discov 2012;11:234-250. PMID:
10.1038/nrd3669. PMID:
22378270.
9.
Boyce BF, Xiu Y, Li J, Xing L, Yao Z. NF-kappaB-mediated regulation of osteoclastogenesis. Endocrinol Metab. 2015; 30: 35-44.

Boyce BF, Xiu Y, Li J, Xing L, Yao Z. NF-kappaB-mediated regulation of osteoclastogenesis.
Endocrinol Metab 2015;30:35-44.
10.
Kim JH, Kim N. Regulation of NFATc1 in osteoclast differentiation. J Bone Metab. 2014; 21: 233-241.

Kim JH, Kim N. Regulation of NFATc1 in osteoclast differentiation.
J Bone Metab 2014;21:233-241. PMID:
10.11005/jbm.2014.21.4.233. PMID:
25489571.
11.
Kim JH, Kim N. Signaling pathways in osteoclast differentiation. Chonnam Med J. 2016; 52: 12-17.

Kim JH, Kim N. Signaling pathways in osteoclast differentiation.
Chonnam Med J 2016;52:12-17. PMID:
10.4068/cmj.2016.52.1.12. PMID:
26865996.
12.
Christenson RH. 1997. Biochemical markers of bone metabolism: An overview. Clin Biochem. 1997; 30: 573-593.

Christenson RH. 1997. Biochemical markers of bone metabolism: An overview.
Clin Biochem 1997;30:573-593. PMID:
9455610.
13.
Lupsa BC, Insogna K. Bone health and osteoporosis. Endocrinol Metab Clin North Am. 2015; 44: 517-530.

Lupsa BC, Insogna K. Bone health and osteoporosis.
Endocrinol Metab Clin North Am 2015;44:517-530. PMID:
26316240.
14.
Diab DL. Watts NB. Postmenopausal osteoporosis. Curr Opin Endocrinol Diabetes Obes. 2013; 20: 501-509.

Diab DL, Watts NB. Postmenopausal osteoporosis.
Curr Opin Endocrinol Diabetes Obes 2013;20:501-509. PMID:
24150190.
15.
Yasui T, Uemura H, Takikawa M, Irahara M. Hormone replacement therapy in postmenopausal women. J Med Invest. 2003; 50: 136-45.
Yasui T, Uemura H, Takikawa M, Irahara M. Hormone replacement therapy in postmenopausal women.
J Med Invest 2003;50:136-45. PMID:
13678382.
16.
Yoo JH. The Life Habits and Exercise Strategies for Prevention and Management of Osteoporosis. Journal of the Korea Entertainment Industry Association(JKEIA). 2016; 10: 137-146.

Yoo JH. The Life Habits and Exercise Strategies for Prevention and Management of Osteoporosis.
Journal of the Korea Entertainment Industry Association(JKEIA) 2016;10:137-146.
17.
Hind K, Burrows M. Weight-bearing exercise and bone mineral accrual in children and adolescents: A review of controlled trials. Bone. 2007; 40: 14-27.

Hind K, Burrows M. Weight-bearing exercise and bone mineral accrual in children and adolescents: A review of controlled trials.
Bone 2007;40:14-27. PMID:
10.1016/j.bone.2006.07.006. PMID:
16956802.
18.
Clark RK. Anatomy and physiology, Jone and Bartlett Publishers. 2005.
Clark RKAnatomy and physiology. Jone and Bartlett Publishers. 2005.
19.
Sun X, Li F, Ma X, Ma J, Zhao B, Zhang Y, Li Y, Lv J, Meng X. The Effects of Combined Treatment with Naringin and Treadmill Exercise on Osteoporosis in Ovariectomized Rats. Sci Rep. 2015; 11: 13009.


Sun X, Li F, Ma X, Ma J, Zhao B, Zhang Y, Li Y, Lv J, Meng X. The Effects of Combined Treatment with Naringin and Treadmill Exercise on Osteoporosis in Ovariectomized Rats.
Sci Rep 2015;11:13009.
20.
Miyatake K, Muneta T, Ojima M, Yamada J, Matsukura Y, Abula K, Sekiya I, Tsuji K. Coordinate and synergistic effects of extensive treadmill exercise and ovariectomy on articular cartilage degeneration. BMC Musculoskelet Disord. 2016; 31: 238.


Miyatake K, Muneta T, Ojima M, Yamada J, Matsukura Y, Abula K, Sekiya I, Tsuji K. Coordinate and synergistic effects of extensive treadmill exercise and ovariectomy on articular cartilage degeneration.
BMC Musculoskelet Disord 2016;31:238.
21.
Blustein JE, Mclaughlin M, Hoffman JR. Exercise effects stress-induced analgesia and spatial learning in rats. Physiol Behav. 2006; 89: 582-586.

Blustein JE, Mclaughlin M, Hoffman JR. Exercise effects stress-induced analgesia and spatial learning in rats.
Physiol Behav 2006;89:582-586. PMID:
10.1016/j.physbeh.2006.07.017. PMID:
16945396.
22.
Kaux JF, Drion P, Libertiaux V, Colige A, Hoffmannn A, Nusgens B, Besancon B, Forthomme B, Le Goff C, Franzen R, Defraigne JO, Cescotto S, Rickert M, Crielaard JM & Croisier JL. Eccentric training improves tendon biomechanical properties: a rat model. J Orthop Res. 2013; 31: 119-124.

Kaux JF, Drion P, Libertiaux V, Colige A, Hoffmannn A, Nusgens B, Besancon B, Forthomme B, Le Goff C, Franzen R, Defraigne JO, Cescotto S, Rickert M, Crielaard JM, et al, Croisier JL. Eccentric training improves tendon biomechanical properties: a rat model.
J Orthop Res 2013;31:119-124. PMID:
10.1002/jor.22202. PMID:
22847600.
23.
Lewis EJH, Ramsook AH, Locke M, Amara CE. Mild eccentirc exercise increases Hsp72 content in skeletal muscles from adult and late middle-aged rats. Cell Stress Chaperones. 2013; 18: 667-673.


Lewis EJH, Ramsook AH, Locke M, Amara CE. Mild eccentirc exercise increases Hsp72 content in skeletal muscles from adult and late middle-aged rats.
Cell Stress Chaperones 2013;18:667-673. PMID:
10.1007/s12192-013-0412-4. PMID:
23443989.
24.
Ogura Y, Naito H, Kakigi R, Ichinoseki-Sekine N, Kurosaka M, Yoshihara T. Akema T. Effects of ageing and endurance exercise training on alpha-actinin isoforms in rat plantaris muscle. Acta Physiol. 2011; 202: 683-690.

Ogura Y, Naito H, Kakigi R, Ichinoseki-Sekine N, Kurosaka M, Yoshihara T, Akema T. Effects of ageing and endurance exercise training on alpha-actinin isoforms in rat plantaris muscle.
Acta Physiol 2011;202:683-690. PMID:
10.1111/j.1748-1716.2011.02284.x.
25.
Kim MH, Jung KS, Nam KH, Jang HJ, Lee SW, Kim YS, Park CS, Lee TH, Park JH, Choi JH, Rho MC, Oh HM. Salvia plebeia R.Br. inhibits signal transduction of IL-6 and prevents ovariectomy-induced bone loss by suppressing osteoclastogenesis. Arch Pharm Res. 2016; 39: 1671-1681.


Kim MH, Jung KS, Nam KH, Jang HJ, Lee SW, Kim YS, Park CS, Lee TH, Park JH, Choi JH, Rho MC, Oh HM. Salvia plebeia R.Br. inhibits signal transduction of IL-6 and prevents ovariectomy-induced bone loss by suppressing osteoclastogenesis.
Arch Pharm Res 2016;39:1671-1681. PMID:
10.1007/s12272-016-0810-0. PMID:
27539608.
26.
Song MY, Wang J, Lee Y, Lee J, Kwon KS, Bae EJ, Park BH. Enhanced M2 macrophage polarization in high n-3 polyunsaturated fatty acid transgenic mice fed a high-fat diet. Mol Nutr Food Res. 2016; 60: 2481-2492.

Song MY, Wang J, Lee Y, Lee J, Kwon KS, Bae EJ, Park BH. Enhanced M2 macrophage polarization in high n-3 polyunsaturated fatty acid transgenic mice fed a high-fat diet. Mol.
Nutr Food Res 2016;60:2481-2492.
27.
Mass├® PG, Tranchant CC, Dosy J, Donovan SM. Coexistence of osteoporosis and cardiovascular disease risk factors in apparently healthy, untreated postmenopausal women. Int J Vitam Nutr Res. 2005; 75: 97-106.

Mass├® PG, Tranchant CC, Dosy J, Donovan SM. Coexistence of osteoporosis and cardiovascular disease risk factors in apparently healthy, untreated postmenopausal women.
Int J Vitam Nutr Res 2005;75:97-106. PMID:
10.1024/0300-9831.75.2.97. PMID:
15929631.
28.
Wilmore JH, Costill DL. Physioligy of sport and exercise(2nd). Human Kinetics, IL: Champaign. 1999.
Wilmore JH, Costill DL. Physioligy of sport and exercise(2nd). Human Kinetics.
IL: Champaign 1999.
29.
Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res. 2011; 15: 6-30.

Cao JJ. Effects of obesity on bone metabolism.
J Orthop Surg Res 2011;15:6-30.
30.
Kim KJ. Effects of soy protein supplementation and treadmill running exercise on the changes of body composition, blood metabolic markers, estradiol, estrogen receptor gene expression in ovariectomized rats. Exercise Sci. 2012; 21: 243-254.

Kim KJ. Effects of soy protein supplementation and treadmill running exercise on the changes of body composition, blood metabolic markers, estradiol, estrogen receptor gene expression in ovariectomized rats.
Exercise Sci 2012;21:243-254.
31.
Suk MH, Jung DC, Shin YA. Effect of Resistance Training on Body Composition, Bone Mineral Density and Adipocytokine in Obese Perimenopausal Women. Korean J Sports Sci. 2009; 20: 693-703.

Suk MH, Jung DC, Shin YA. Effect of Resistance Training on Body Composition, Bone Mineral Density and Adipocytokine in Obese Perimenopausal Women.
Korean J Sports Sci 2009;20:693-703.
32.
Li L, Chen X, Lv S, Dong M, Zhang L, Tu J, Yang J, Zhang L, Song Y, Xu L, Zou J. Influence of exercise on bone remodeling-related hormones and cytokines in ovariectomized rats: a model of postmenopausal osteoporosis. PLoS One. 2014; 13(11): e112845.

Li L, Chen X, Lv S, Dong M, Zhang L, Tu J, Yang J, Zhang L, Song Y, Xu L, Zou J. Influence of exercise on bone remodeling-related hormones and cytokines in ovariectomized rats: a model of postmenopausal osteoporosis.
PLoS One 2014;13(11):e112845PMID:
10.1371/journal.pone.0112845.
33.
Scott JP, Sale C, Greeves JP, Casey A, Dutton J, Fraser WD. Treadmill running reduces parathyroid hormone concentrations during recovery compared with a nonexercising control group. J Clin Endocrinol Metab. 2014; 99: 1774-1782.


Scott JP, Sale C, Greeves JP, Casey A, Dutton J, Fraser WD. Treadmill running reduces parathyroid hormone concentrations during recovery compared with a nonexercising control group.
J Clin Endocrinol Metab 2014;99:1774-1782. PMID:
10.1210/jc.2013-3027. PMID:
24476072.
34.
Stringhetta-Garcia CT, Singulani MP, Santos LF, Louzada MJ, Nakamune AC, Chaves-Neto AH, Rossi AC, Ervolino E, Dornelles RC. The effects of strength training and raloxifene on bone health in aging ovariectomized rats. Bone. 2016; 85: 45-54.

Stringhetta-Garcia CT, Singulani MP, Santos LF, Louzada MJ, Nakamune AC, Chaves-Neto AH, Rossi AC, Ervolino E, Dornelles RC. The effects of strength training and raloxifene on bone health in aging ovariectomized rats.
Bone 2016;85:45-54. PMID:
10.1016/j.bone.2015.11.023. PMID:
26812611.
35.
JAMA. NIH consensus conference: Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001; 285: 785-95.
JAMA. NIH consensus conference: Osteoporosis prevention, diagnosis, and therapy.
JAMA 2001;285:785-95. PMID:
11176917.
36.
Sarioglu M, Tuzun C, Unlu Z, Tikiz C, Taneli F, Uyanik BS. Comparison of the effects of alendronate and risedronate on bone mineral density and bone turnover markers in postmenopausal osteoporosis. Rheumatol Int. 2006; 26: 195-200.


Sarioglu M, Tuzun C, Unlu Z, Tikiz C, Taneli F, Uyanik BS. Comparison of the effects of alendronate and risedronate on bone mineral density and bone turnover markers in postmenopausal
osteoporosis.
Rheumatol Int 2006;26:195-200. PMID:
10.1007/s00296-004-0544-z. PMID:
15580349.
37.
Shearer MJ. Role of vitamin K and Gla proteins in the pathophysiology of osteoporosis and vascular calcification. Curr Opin Clin Nutr Metab Care. 2000; 3: 433-438.

Shearer MJ. Role of vitamin K and Gla proteins in the pathophysiology of osteoporosis and vascular calcification.
Curr Opin Clin Nutr Metab Care 2000;3:433-438. PMID:
10.1097/00075197-200011000-00004. PMID:
11085828.
38.
Lim SK. Clinical significance and application of bone turnover marker. Korean J Bone Metabolism. 1994; 1: 1-11.
Lim SK. Clinical significance and application of bone turnover marker.
Korean J Bone Metabolism 1994;1:1-11.
39.
Tartibian B, Hajizadeh Maleki B, Kanaley J, Sadeghi K. Long-term aerobic exercise and omega-3 supplementation modulate osteoporosis through inflammatory mechanisms in post-menopausal women: a randomized, repeated measures study. Nutr Metab (lond). 2011; 15: 71-83.

Tartibian B, Hajizadeh Maleki B, Kanaley J, Sadeghi K. Long-term aerobic exercise and omega-3 supplementation modulate osteoporosis through inflammatory mechanisms in post-menopausal women: a randomized, repeated measures study.
Nutr Metab (lond) 2011;15:71-83.
40.
Iwamoto J, Shimamura C, Takeda T, Abe H, Ichimura S, Sato Y, Toyama Y. Effects of treadmill exercise on bone mass, bone metabolism, and calciotropic hormones in young growing rats. J Bone Miner Metab. 2004; 22: 26-31.


Iwamoto J, Shimamura C, Takeda T, Abe H, Ichimura S, Sato Y, Toyama Y. Effects of treadmill exercise on bone mass, bone metabolism, and calciotropic hormones in young growing rats.
J Bone Miner Metab 2004;22:26-31. PMID:
10.1007/s00774-003-0443-5. PMID:
14691683.
41.
Jung HY. Comparative analysis of body mass index and body mineral density of female college students at their 20sŌĆÖ according to the existence of physical exercise. 2004; Ewha Womans University.
Jung HYComparative analysis of body mass index and body mineral density of female college students at their 20sŌĆÖ according to the existence of physical exercise. 2004. Ewha Womans University.
42.
Ross R, Ringer J, Diamond L, Fenton F. Identifying and treating Osteoporosis in Women. Physician Assist. 2001; 25: 40-47.
Ross R, Ringer J, Diamond L, Fenton F. Identifying and treating Osteoporosis in Women.
Physician Assist 2001;25:40-47.
43.
Kwan P. Osteoporosis: from osteoscience to neuroscience and beyond. Mech Ageing Dev. 2015; 145: 26-38.

Kwan P. Osteoporosis: from osteoscience to neuroscience and beyond.
Mech Ageing Dev 2015;145:26-38. PMID:
10.1016/j.mad.2015.02.001. PMID:
25681683.
44.
Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res. 2000; 15: 2-12.

Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption.
J Bone Miner Res 2000;15:2-12. PMID:
10.1359/jbmr.2000.15.1.2. PMID:
10646108.
45.
Ju YI, Sone T, Ohnaru K, Tanaka K, Fukunaga M. Effect of swimming exercise on three-dimensional trabecular bone microarchitecture in ovariectomized rats. J Appl Physiol. 2015; 119: 990-997.

Ju YI, Sone T, Ohnaru K, Tanaka K, Fukunaga M. Effect of swimming exercise on three-dimensional trabecular bone microarchitecture in ovariectomized rats.
J Appl Physiol 2015;119:990-997. PMID:
10.1152/japplphysiol.00147.2015. PMID:
26338454.
46.
Soysa NS, Alles N, Shimokawa H, Jimi E, Aoki K, Ohya K. Inhibition of the classical NF-kappaB pathway prevents osteoclast bone-resorbing activity. J Bone Miner Metab. 2009; 27: 131-139.


Soysa NS, Alles N, Shimokawa H, Jimi E, Aoki K, Ohya K. Inhibition of the classical NF-kappaB pathway prevents osteoclast bone-resorbing activity.
J Bone Miner Metab 2009;27:131-139. PMID:
19172225.
47.
Ikeda F, Nishimura R, Matsubara T, Hata K, Reddy SV, Yoneda T. 2006. Activation of NFAT signal in vivo leads to osteopenia associated with increased osteoclastogenesis and bone-resorbing activity. J Immunol. 2006; 177: 2384-2390.

Ikeda F, Nishimura R, Matsubara T, Hata K, Reddy SV, Yoneda T. 2006. Activation of NFAT signal in vivo leads to osteopenia associated with increased osteoclastogenesis and bone-resorbing activity.
J Immunol 2006;177:2384-2390. PMID:
16888000.
48.
Wang Q, Yang M, Wang J. Effects of treadmill exercise on mRNA expression levels of osteoprotegerin, RANKL and RUNX2 in bone tissues of ovariectomized. Zhongguo Gu Shang. 2013; 26: 940-943.
Wang Q, Yang M, Wang J. Effects of treadmill exercise on mRNA expression levels of osteoprotegerin, RANKL and RUNX2 in bone tissues of ovariectomized.
Zhongguo Gu Shang 2013;26:940-943. PMID:
24605748.