INTRODUCTION
Regular exercise results in physiological adaptations that benefit almost every organ system in the body to improve overall health. Endurance exercise has long been reported to decrease obesity, ameliorate the postprandial triglyceridemic response, increase the fat oxidation rate, and improve insulin sensitivity [1-4].
Conjugated linoleic acid (CLA) is an important food component that consists of a mixture of positional and geometric isomers of linoleic acid (cis-9, cis-12, and C18:2) with two conjugated double bonds at various carbon positions in the fatty acid chain [5]. A mixed CLA isomer is commercially marketed as a weight-loss supplement. Although CLA is considered an alternative medicine with minimal side effects compared with mainstream anti-obesity drugs, it has been reported to induce upset stomach, nausea, and loose stools because of its lack of absorption and instability [6,7]. CLA is also considered an important biologically-active compound in food due to its proven anti-carcinogenic, anti-allergic, and anti-inflammatory properties [8-10]. Feeding studies in animals have suggested that a 0.4-2.0% CLA mixture [5-10] can be used to determine the effect of a mixed CLA isomer. Based on these studies, was used a 1.0% CLA mixture as a supplement in this study.
Leptin and insulin levels increase as a result of obesity [11]. Leptin exerts central effects on hypothalamic-pituitary function and markedly regulates appetite, thermogenesis, and food intake [12]. Dietary intake and body weight are challenged by the fact that the majority of obese people exhibit high levels of circulating leptin, to which they are apparently resistant [13]. Insulin is released from the pancreas and leptin is released from adipose tissue, and these hormones help regulate long-term energy balance [14]. Recent experimental data suggest that leptin can act directly on the liver to influence the insulin and leptin signaling pathways [15].
Adipose tissue is active, and secretes proteins, such as leptin, interleukin (IL)-6, IL-8, and tumor necrosis factor (TNF)-α, which play key roles regulating cellular and humoral immune responses to malignancies [16,17]. An increase in IL-6 level combined with detectable levels of IL-1β result in a significantly elevated risk for developing type 2 diabetes that is not observed when either cytokine alone is detected at a higher level [18]. IL-1β has similar actions to those of IL-6 and TNF-α, and IL-1β and TNF-α are potent inducers of IL-6 production and activity. In contrast, IL-6 may inhibit IL-1β and TNF-α production and inhibit TNF-mediated monocyte cytotoxicity to tumor target cells [19].
CLA is considered a weight-loss supplement, and endurance exercise decreases the frequency of obesity. Therefore, we compared the effects of endurance exercise on the levels of appetite-regulating hormones, such as leptin and insulin, as well as pro-inflammatory cytokines in rats supplemented with 1.0% CLA.
METHODS
Experimental animals and dietary intake measurement
Six-week-old male Sprague-Dawley rats (weight, 230-250 g) were obtained (Samtako Co., Osan, Korea) and housed individually in a controlled environment of 23±1°C, 50±5% relative humidity, and a 12 hour light-dark cycle. All animals were given free access to tap water and food. After the 1-week acclimation period, the rats were divided randomly into three groups: HS, high-fat diet (HFD) (35% fat of total diet weight) [20] sedentary; CS, CLA supplemented HFD sedentary group; and CE, CLA supplemented HFD exercise group. CLA (1.0% of a 76.82% CLA mixture; 36.8% cis-9,trans-11 CLA, 37.8% trans-10,cis-12 CLA, and 1.2% trans-9,trans-11 CLA) (HK Biotech Co., Gyeongnam, Korea) was substituted for the dietary fat in the adjusted HFD (Table 1). Food intake was measured daily, and the change in body weight of each animal was noted weekly. The diets were modified from a previous study [20] and based on AIN-76G. The food efficiency ratio was calculated as total weight gain divided by total food intake for the experimental period. All experimental protocols were approved by the Animal Study Committee of Sunmoon University.
Table 1.
Composition of the experimental diets
| Variable | High-fat diet1) | CLA Suppl. diet2) |
|---|---|---|
| Ingredients (g/kg diet) | ||
| Casein | 200.0 | 200.0 |
| Starch | 200.0 | 200.0 |
| Sucrose | 150.0 | 150.0 |
| Lard | 350.0 | 337.0 |
| Cellulose | 50.0 | 50.0 |
| Mineral mix | 35.0 | 35.0 |
| Vitamin mix | 10.0 | 10.0 |
| DL-methionine | 3.0 | 3.0 |
| Choline barbiturate | 2.0 | 2.0 |
| DL-a-tocopherol | 1.2 | 1.2 |
| c-9, t-11 CLA3) | - | 6.5 |
| t-10, c-12 CLA3) | - | 6.5 |
| Energy (kcal/kg) | 5350.0 | 5350.0 |
| Protein (% kcal/g) | 15.0 | 15.0 |
| Carbohydrate (% kcal/g) | 26.0 | 26.0 |
| Fat (% kcal/g) | 59.0 | 59.0 |
Exercise protocol and sample collection
The exercised rats swam (50-60% VO2max) 60 min/day, 5 days/week for 4 weeks [2,21]. Water temperature in the plastic barrel (depth, 50 cm and radius, 25 cm) swimming pool was maintained at 35±1°C [22]. At the end of the experiment, the 12-hour overnight-fasted rats were sacrificed by exsanguination, and blood was withdrawn from the left ventricle under light diethyl ether anesthesia. Serum, liver, gastrocnemius muscle, and perirenal fat were dissected and immediately snap-frozen in liquid nitrogen. The organs and tissue were stored at -70°C until analyses.
Biochemical assays
Fasting serum insulin levels were measured using standard radioimmunoassay kits (Linco Research, Inc., St. Louis, MO, USA). Serum leptin, IL-1β, and IL-6 levels were analyzed using quantitative sandwich enzyme immunoassay kits (R & D Systems, San Diego, CA, USA). The lower limit of detection was 3.13 pg/ml. Values below this limit were assumed to be zero for the statistical analysis. The inter- and intra-assay coefficients of variation were <10%.
Statistical analysis
Data are expressed as mean±standard error using the SPSS/PC ver. 18.0 software program for Windows (SPSS Inc., Chicago, IL, USA). All statistical analyses were conducted using one-way analysis of variance followed by the least significant difference post-hoc test. Linear relationships between leptin and insulin levels and IL-1β and IL-6 levels were examined using Pearson’s correlation analysis. A p<0.05 was considered significant.
RESULTS
Changes in the body composition-related variables
The changes in body composition-related variables are summarized in Table 2. Total body weight and cumulative body weight gain in the CS and CE groups decreased significantly compared to those in the HS group (p<0.001). Liver and perirenal fat weights in the CS (p<0.05) and CE (p<0.01) groups decreased significantly compared to those in the HS group. However, gastrocnemius muscle weight was not different among the groups.
Table 2.
Body composition-related variables
| Variable (g) | HS (n=8) | CS (n=8) | CE (n=8) |
|---|---|---|---|
| Body weight | 467.9±3.3 | 432.0±5.6*** | 415.2±5.5*** |
| Cumulative body weight gain | 198.1±3.2 | 159.8±3.1*** | 144.2±2.9*** |
| Liver | 11.9±0.2 | 10.5±0.3* | 10.2±0.3** |
| Gastrocnemius muscle | 1.909±0.043 | 1.930±0.074 | 1.995±0.039 |
| Perirenal fat pad | 16.1±1.1 | 13.3±1.4* | 11.1±0.6** |
Changes in appetite-related variables
Serum leptin and insulin levels in the CS and CE groups decreased significantly relative to those in the HS group (p<0.001), whereas leptin (p<0.01) and insulin (p<0.05) levels in the CE group decreased significantly compared to those in the CS group (Table 3). Food intake amounts by the CS and CE groups were significantly lower than that by the HS group (p<0.001). The food efficiency ratio values of the CS (p<0.001) and CE (p<0.001) groups were lower than that of the HS group, whereas the food efficiency ratio of the CE group decreased significantly (p<0.01).
Table 3.
Appetite-related variables
| Variable | HS (n=8) | CS (n=8) | CE (n=8) |
|---|---|---|---|
| Hormonal variables | |||
| Leptin (ng/mL) | 21.4±0.7 | 12.7±0.6*** | 7.8±0.5***,## |
| Insulin (ng/mL) | 4.9±0.5 | 3.0±0.3*** | 1.7±0.4***,# |
| Food intake variables | |||
| Food intake amount (g) | 381.8±3.1 | 340.8±3.8*** | 338.8±7.1*** |
| Food efficiency ratio | 0.216±0.008 | 0.163±0.005*** | 0.119±0.145***,## |
Changes in pro-inflammatory cytokines
Changes in the pro-inflammatory cytokines IL-1β and IL-6 are presented in Table 4. IL-1β levels in the CS and CE groups decreased significantly compare to those in the HS group (p<0.001). In addition, IL-6 levels in the CS and CE groups were significantly lower than those in the CS group (p<0.01).
Linear relationships between leptin and insulin and the pro-inflammatory cytokines
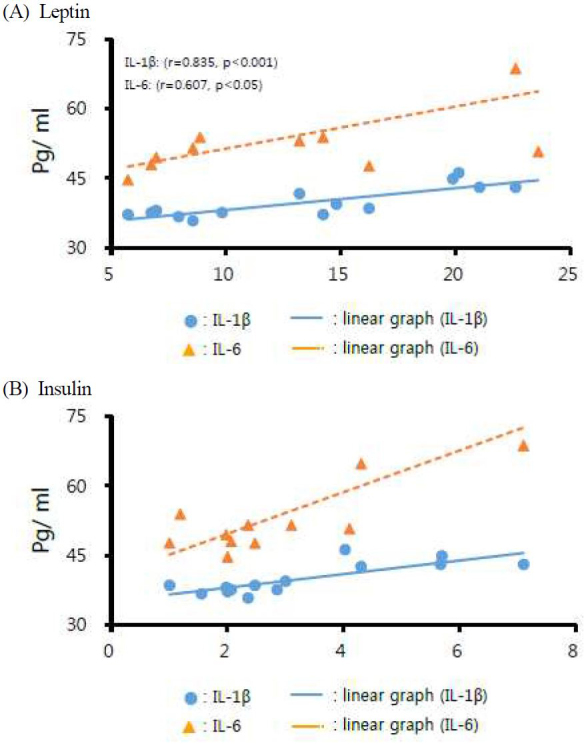
The levels of leptin and the pro-inflammatory cytokines (IL-1β: r=0.835, p<0.001 and IL-6: r=0.607, p<0.05) were positively correlated (Fig. 1). Additionally, highly significant positive correlations were detected between levels of insulin and the pro-inflammatory cytokines (IL-1β: r=0.797, p<0.01 and IL-6: r=0.827, p<0.01).
DISCUSSION
The results of this study show that the CLA diet and endurance exercise were positively associated with the levels of insulin, leptin, and pro-inflammatory cytokines, such as IL-1β and IL-6, in rats fed a high-fat diet.
Endurance exercise enhances weight loss because it lowers the energetic balance by increasing energy expenditure and reducing food intake [1-3]. Haghshenas et al [14] reported that increased energy intake is likely to result from changes in the appetite control system toward an anorexigenic environment. Our findings agree with studies reported previously, showing that regular participation in endurance exercise decreases body and fat mass [23,24].
The most pronounced effect of supplementation with the CLA isomer is stimulation of protein synthesis, which reduces body mass and fat mass [25-30]. Previous studies showed that CLA reduced adiposity in experimental animals, including mice, rats, and pigs [5-10]. These animal studies demonstrate that total fat mass decreased >50% in animals fed a diet containing a 1.0% CLA mixture compared to those fed a control diet (without CLA). Thus, we used 1.0% CLA in an AIN-76-based diet in the present study. As mentioned previously, CLA is marketed as a weight-loss supplement. Our results are consistent with results of previous studies. The rat groups supplemented with CLA with/without endurance exercise tended to have lower cumulative body weight gain and fat pad weight. Thus, our results show that 1.0% CLA supplementation may reduce body and fat weight regardless of endurance exercise while eating a high-fat diet.
Some scholars have considered leptin to be a warning mechanism for adjusting the body’s fat content [31]. Alessia et al. [32] reported that dietary CLA supplementation decreases leptin in porcine adipose tissue, and they hypothesize an increase in noradrenergic stimulation of lipolysis directly in target tissue. Other studies have reported that regular endurance exercise ameliorates serum leptin levels [33]. An impaired hypothalamic insulin signaling pathways is sufficient to promote obesity and type 2 diabetes in different genetic rodent models in which insulin signaling was neuronally ablated [12,14,15]. In our study, insulin levels decreased in the CS and CE groups compared to those in HFD and sedentary groups. These findings show that a CLA-supplemented diet decreased insulin level, but endurance exercise had no effect. Some studies have reported that CLA is inversely associated with serum insulin and leptin levels [34-39]. They assumed that a decrease in insulin concentration suggested that CLA supplementation enhanced insulin sensitivity. Our results were in line with those reports. In our results, insulin and leptin, which are appetite-regulating hormones, showed synergistic effects during endurance exercise, suggesting that endurance exercise could potentially lower leptin and insulin resistance during CLA supplementation. Therefore, combining endurance exercise and CLA supplementation may improve leptin and insulin levels compared to those of CLA supplementation alone, even in rats fed HFD.
Some studies have demonstrated that endurance exercise inhibits release of the inflammatory mediators IL-1β, IL-6, and TNF-α from fat tissues by decreasing the stimulation of the sympathetic system and increasing production of anti- inflammatory cytokines [40-42]. Those reports have described that human leptin stimulates the production of cytokines, such as TNF-α and IL-6. In the present study, we found significant improvement between serum leptin levels and inflammatory markers such as IL-1β and IL-6. Our observation suggests that combining CLA supplementation and endurance exercise may help improve leptin level by controlling pro-inflammatory cytokines, such as IL-1B and IL-6, in adolescent rats fed a HFD.
Leptin and insulin secretion are regulated by inflammatory mediators and exert central effects on hypothalamic-pituitary function [14]. IL-1 and TNF-α have been implicated in the regulation of leptin secretion where they induce production of leptin from adipocytes [43,44]. In addition, Takács et al. reported that human leptin stimulates production of cytokines, such as TNF-α and IL-6 [45]. Our results agree with previous studies that increased leptin and insulin concentrations might lead to increased IL-1β and IL-6 concentrations. Although, there was no synergistic effect of the CLA diet and endurance exercise on IL-1β and IL-6 levels, the appetite-regulating hormones and pro-inflammatory cytokines were significantly positively correlated with IL-1β and IL-6 in our results, suggesting that the leptin and insulin induced by CLA and endurance exercise in rats fed a HFD stimulated production of pro-inflammatory cytokines, such as IL-1β and IL-6.
As mentioned above, combining CLA supplementation and endurance exercise tended to result in lower obesity indices and improved of appetite-regulating hormone and pro-inflammatory cytokine levels than CLA supplementation or endurance exercise alone. We did not test the effect of exercise on the HFD group. Thus, we cannot explain the effect of exercise without CLA supplementation, which is a limitation.
In conclusion, endurance exercise may play a beneficial role with CLA supplementation in rats on a HFD to improve levels of appetite-regulating hormones and pro-inflammatory cytokines.