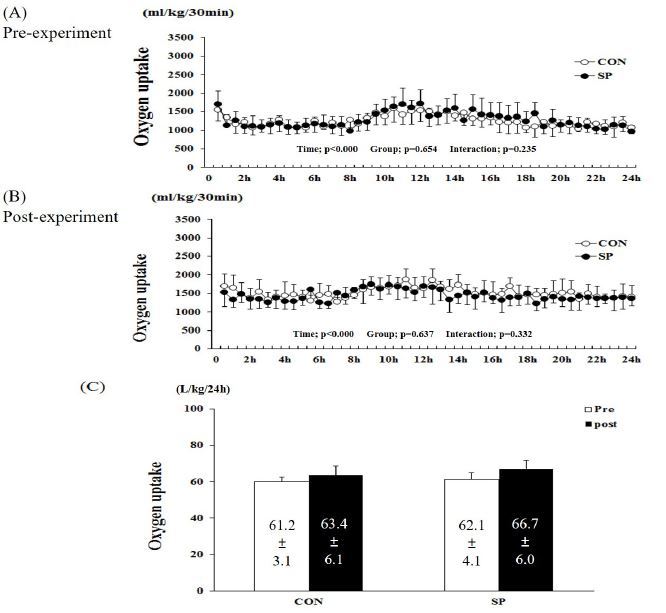
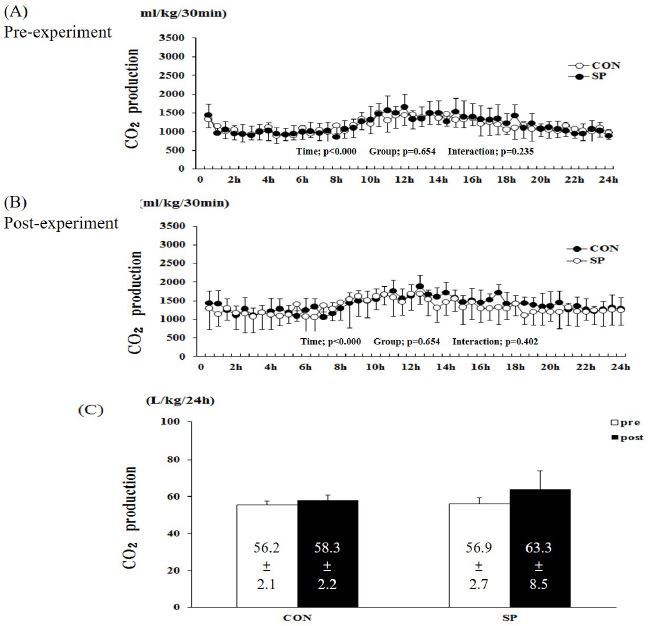
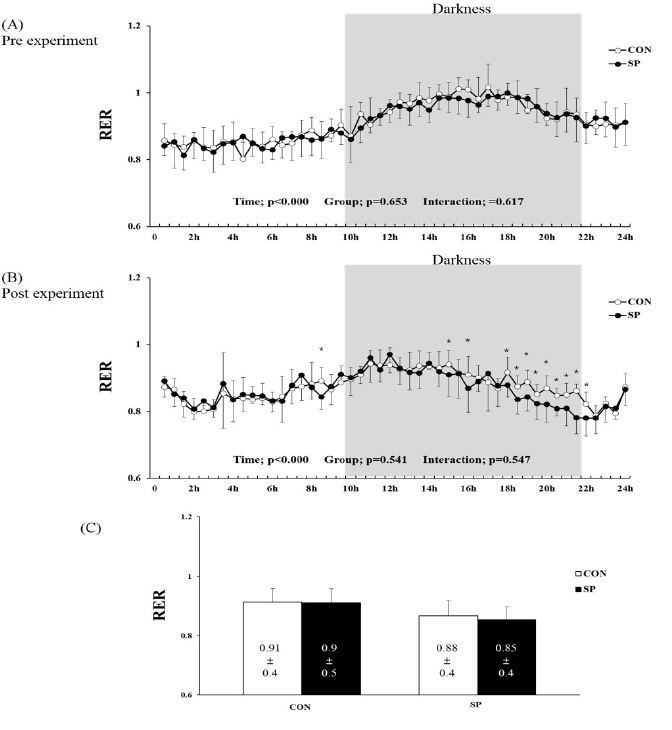
1.
Jung EY, Lee HS, Lee HJ, Kim JM, Lee KW, Suh HJ. Feeding silk protein hydrolysates to C57BL/KsJ-db/db mice improves blood glucose and lipid profiles. Nutr Res. 2010; 30: 783-90.

Jung EY, Lee HS, Lee HJ, Kim JM, Lee KW, Suh HJ. Feeding silk protein hydrolysates to C57BL/KsJ-db/db mice improves blood glucose and lipid profiles.
Nutr Res 2010;30:783-90. PMID:
10.1016/j.nutres.2010.10.006. PMID:
21130298.
2.
Ahmad R, Kamra A, Hasnain SE. Fibroin silk proteins from the nonmulberry silkworm Philosamia ricini are biochemically and immunochemically distinct from those of the mulberry silkworm Bombyx mori. DNA Cell Bio. 2004; 23: 149-54.

Ahmad R, Kamra A, Hasnain SE. Fibroin silk proteins from the nonmulberry silkworm Philosamia ricini are biochemically and immunochemically distinct from those of the mulberry silkworm Bombyx mori.
DNA Cell Bio 2004;23:149-54. PMID:
10.1089/104454904322964742. PMID:
15068584.
3.
Lee SH, Park GY, Bae DK, Yang YH. Silk and silkworm pupa peptides suppress adipogenesis in preadipocytes and fat accumulation in rats fed a high-fat diet. Eur J Nutr. 2012; 51: 1011-9.


Lee SH, Park GY, Bae DK, Yang YH. Silk and silkworm pupa peptides suppress adipogenesis in preadipocytes and fat accumulation in rats fed a high-fat diet.
Eur J Nutr 2012;51:1011-9. PMID:
10.1007/s00394-011-0280-6. PMID:
22160191.
4.
Park DS, Lee SH, Choi YJ, Bae DK, Yang YH, Goeun Y, Kim TK, Yeon SH, Hwang SY, Joo SS, Kim YB. Improving effect of silk peptides on the cognitive function of rats with aging brain facilitated by d-galactose. Biomol Ther. 2011; 19: 224-30.


Park DS, Lee SH, Choi YJ, Bae DK, Yang YH, Goeun Y, Kim TK, Yeon SH, Hwang SY, Joo SS, Kim YB. Improving effect of silk peptides on the cognitive function of rats with aging brain facilitated by d-galactose.
Biomol Ther 2011;19:224-30. PMID:
10.4062/biomolther.2011.19.2.224.
5.
Kim TK, Park DS, Yeon SH, Lee SH, Choi YJ, Bae DK, Yang YH, Yang GE, Joo SS, Lim WT, Lee JY, Lee JS, Jeong HS, Hwang SY. Tyrosine-fortified silk amino acids improve physical function of parkinson’s disease rats. Food Sci Bioteclnol. 2011; 20: 79-84.


Kim TK, Park DS, Yeon SH, Lee SH, Choi YJ, Bae DK, Yang YH, Yang GE, Joo SS, Lim WT, Lee JY, Lee JS, Jeong HS, Hwang SY. Tyrosine-fortified silk amino acids improve physical function of parkinson’s disease rats.
Food Sci Bioteclnol 2011;20:79-84. PMID:
10.1007/s10068-011-0011-z.
6.
Kim DW, Hwang HS, Kim DS, Sheen SH, Heo DH, Hwang G, Kang SH, Kweon H, Jo YY, Kang SW, Lee KG, Park J, Eum WS, Cho YJ, Choi SY. Enhancement of anti-inflammatory activity of PEP-1-FK506 binding protein by silk fibroin peptide. J Microbiol Biotechnol. 2012; 22: 494-500.

Kim DW, Hwang HS, Kim DS, Sheen SH, Heo DH, Hwang G, Kang SH, Kweon H, Jo YY, Kang SW, Lee KG, Park J, Eum WS, Cho YJ, Choi SY. Enhancement of anti-inflammatory activity of PEP-1-FK506 binding protein by silk fibroin peptide.
J Microbiol Biotechnol 2012;22:494-500. PMID:
10.4014/jmb.1111.11024. PMID:
22534296.
7.
Noriaki N, Takatoshi M, Yoshimasa I, Norio O, Masahiro S. Enhancing effects of sericin on corneal wound healing in otsuka longevans thkushima fatty rats as a model of human type 2 diabetes. Biol Pharm Bull. 2009; 32: 1594-9.

Noriaki N, Takatoshi M, Yoshimasa I, Norio O, Masahiro S. Enhancing effects of sericin on corneal wound healing in otsuka longevans thkushima fatty rats as a model of human type 2 diabetes.
Biol Pharm Bull 2009;32:1594-9. PMID:
10.1248/bpb.32.1594. PMID:
19721238.
8.
Hwang EH, Kang BG, Kim BY, Lee HJ. Protein quality evaluation and effect of plasma lipid contents of acid hydrolysates of cocoon in rats fed by high cholesterol, high triglyceride and high sucrose diet. J Kor Soc Food Sci Nutr. 2001; 30: 1004-9.
Hwang EH, Kang BG, Kim BY, Lee HJ. Protein quality evaluation and effect of plasma lipid contents of acid hydrolysates of cocoon in rats fed by high cholesterol, high triglyceride and high sucrose diet.
J Kor Soc Food Sci Nutr 2001;30:1004-9.
9.
Jung EY, Lee HS, Lee YJ, Kim JM, Lee K, Suh HJ. Feeding silk protein hydrolysates to C57BL/KsJ-db/db mice improves blood glucose and lipid profile. Nutr Res. 2010; 30: 783-90.

Jung EY, Lee HS, Lee YJ, Kim JM, Lee K, Suh HJ. Feeding silk protein hydrolysates to C57BL/KsJ-db/db mice improves blood glucose and lipid profile.
Nutr Res 2010;30:783-90. PMID:
10.1016/j.nutres.2010.10.006. PMID:
21130298.
10.
Shin SH, Park DS, Yeon SH, Jeon JH, Kim TK, Joo SS, Lim WT, Lee JY, Kim YB. Stamina-enhancing effects of silk amino acid preparations in mice. Lab Anim Res. 2009; 25: 127-34.
Shin SH, Park DS, Yeon SH, Jeon JH, Kim TK, Joo SS, Lim WT, Lee JY, Kim YB. Stamina-enhancing effects of silk amino acid preparations in mice.
Lab Anim Res 2009;25:127-34.
11.
Lee YS, Park MJ, Choi JE, Kim JY, Nam MS, Jeong YH. Effects of Silk Protein Hydrolysates on Blood Glucose Level, Serum Insulin and Leptin Secretion in OLETF Rats. J Kor Soc Food Sci Nutr. 2007; 36: 703-7.


Lee YS, Park MJ, Choi JE, Kim JY, Nam MS, Jeong YH. Effects of Silk Protein Hydrolysates on Blood Glucose Level, Serum Insulin and Leptin Secretion in OLETF Rats.
J Kor Soc Food Sci Nutr 2007;36:703-7. PMID:
10.3746/jkfn.2007.36.6.703.
12.
Lee MS, Kim DM, Cho BN, Koo SJ, Jew SS, Jin DK, Lee SH. Study on consequent body fat and serum lipid metabolism after cocoon hydrolysate, green tea leaves and dietary fiber supplementation. J Korean Soc Chem Biotechnol. 2003; 46: 123-9.
Lee MS, Kim DM, Cho BN, Koo SJ, Jew SS, Jin DK, Lee SH. Study on consequent body fat and serum lipid metabolism after cocoon hydrolysate, green tea leaves and dietary fiber supplementation.
J Korean Soc Chem Biotechnol 2003;46:123-9.
13.
Kim JS, Hwang HJ, Yun HY, Kim BK, Lee CH, Suh HJ, Lim KW. Silk Peptide intake increases fat oxidation at rest in exercised mice. J Nutr Sci Vitaminol. 2013; 59: 250-5.

Kim JS, Hwang HJ, Yun HY, Kim BK, Lee CH, Suh HJ, Lim KW. Silk Peptide intake increases fat oxidation at rest in exercised mice.
J Nutr Sci Vitaminol 2013;59:250-5. PMID:
10.3177/jnsv.59.250. PMID:
23883697.
14.
Kim JS, Hwang HJ, Park JH, Yun HY, Suh HJ, Lim KW. Silk peptide treatment can improve the exercise performance of mice. J Int Soc Sports Nutr. 2014; 11: 35

. Kim JS, Hwang HJ, Park JH, Yun HY, Suh HJ, Lim KW. Silk peptide treatment can improve the exercise performance of mice.
J Int Soc Sports Nutr 2014;11:35PMID:
10.1186/1550-2783-11-35. PMID:
25050085.
15.
Lim KW, Kim JS, Jeon YR, Hwang HJ, Suh HJ. Measurement of resting metabolic rate using metabolic chamber in resting rats. J Exerc Nutr Biochem. 2011; 15: 35-40.

Lim KW, Kim JS, Jeon, YR, Hwang, HJ, Suh, HJ. Measurement of resting metabolic rate using metabolic chamber in resting rats.
J Exerc Nutr Biochem 2011;15:35-40.
16.
Kim JS, Jeon YR, Hwang HJ, Suh HJ and Lim KW. Effects of oral caffeine and capsaicin administration on energy expenditure and energy substrates utilization in resting rats. J Exerc Nutr Biochem. 2011; 15: 183-9.

Kim JS, Jeon YR, Hwang HJ, Suh HJ, et al, Lim KW. Effects of oral caffeine and capsaicin administration on energy expenditure and energy substrates utilization in resting rats.
J Exerc Nutr Biochem 2011;15:183-9.
17.
Park KJ, Hong SE, Do MS, Hyun CK. Stimulation of insulin secretion by silk fibroin hydrolysate in streptozotocin-induced diabetic rats and db/db mice. Kor J Pharmacogn. 2002; 33: 21-8.
Park KJ, Hong SE, Do MS, Hyun CK. Stimulation of insulin secretion by silk fibroin hydrolysate in streptozotocin-induced diabetic rats and db/db mice.
Kor J Pharmacogn 2002;33:21-8.
18.
Bonen A, Cambell S, Benton C, Chabowski A, Coort S, Han Z, Koonen D, Glatz J, Luiken J. Regulation of fatty acid transport by fatty acid translocase/CD36. proc Nutrition Soc. 2004; 63: 245-9.

Bonen A, Cambell S, Benton C, Chabowski A, Coort S, Han Z, Koonen D, Glatz J, Luiken J. Regulation of fatty acid transport by fatty acid translocase/CD36.
proc Nutrition Soc 2004;63:245-9. PMID:
10.1079/PNS2004331. PMID:
15294038.