INTRODUCTION
METHODS
Animals
Training protocol and medication
Tissue collection
Measurement of bone mineral density (BMD) and bone strength
Micro-computed tomography (Micro-CT)
Serum insulin concentration
Serum Gla-osteocalcin concentration
Serum Glu-osteocalcin concentration
RESULTS
Body mass and composition (Table 1).
Table 1.
Body mass and composition
|
S n=6 |
SE n=6 |
O n=10 |
OE n=11 |
OE2 n=6 |
OEE n=6 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Final wt (g) | 296.7 | 10.96 | 289.3cdf | 6.05 | 366abdef | 8.87 | 333.9abcef | 6.68 | 273.3cd | 3.62 | 242.3abcd | 9.94 |
| Food intake (g/day) | 17.4 | 0.62 | 15.8 | 0.54 | 19.0 | 0.97 | 17.3 | 0.50 | 16.2 | 0.89 | 16.7 | 1.80 |
| UW (g) | 0.62cd | 0.06 | 0.64cd | 0.10 | 0.13abef | 0.01 | 0.10abef | 0.01 | 0.62cd | 0.04 | 0.60cd | 0.04 |
| UW/kg wt | 0.21cd | 0.02 | 0.23cd | 0.03 | 0.04abef | 0.00 | 0.03abef | 0.00 | 0.24cd | 0.01 | 0.25cd | 0.03 |
| FW (g) | 8.92c | 1.94 | 6.79c | 0.81 | 14.54abdef | 1.37 | 8.77cf | 1.01 | 5.39cd | 0.93 | 2.92cd | 1.05 |
| FW/kg wt | 3.01f | 0.61 | 2.44c | 0.26 | 4.10bdef | 0.33 | 2.73cf | 0.29 | 2.07c | 0.35 | 1.24acd | 0.38 |
| Soleus/kg wt | 0.04 | 0.00 | 0.04 | 0.00 | 0.03 | 0.00 | 0.04 | 0.01 | 0.04 | 0.00 | 0.04 | 0.00 |
S, sham-operated and sedentary; SE, sham-operated and exercise trained; O, ovariectomized and sedentary; OE, ovariectomized and exercise trained; OE2, ovariectomized and given 17β-estradiol; OEE, ovariectomized, exercise trained, and given 17β-estradiol; Wt, weight; UW, uterine weight; FW, fat weight
Bone volume, BMD, and breaking strength of the femur and tibia (Table 2 and Fig. 1)
Figure 1.
High-intensity swimming training protocol Number of repetitions per set: 14. The rats performed 20-s swimming bouts with a weight of a weight equivalent to 14, 15, and 16% of body weight for the first 5, the next 9, and the last 5 days, respectively.
Table 2.
Bone weight, bone mineral density (BMD), and breaking strength of the femur (F) and tibia (T)
|
S n=6 |
SE n=6 |
O n=10 |
OE n=11 |
OE2 n=6 |
OEE n=6 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Femur/kg wt (g) | 0.27cf | 0.01 | 0.30c | 0.01 | 0.23abdef | 0.00 | 0.27cf | 0.01 | 0.30cf | 0.01 | 0.33acde | 0.01 |
| Tibia/kg wt (g) | 0.22cf | 0.01 | 0.25c | 0.01 | 0.19abdef | 0.00 | 0.23cf | 0.00 | 0.25c | 0.01 | 0.27acd | 0.01 |
| F-BMD/kg wt (mg/cm2/kg) | 0.08c | 0.00 | 0.09cd | 0.00 | 0.07abef | 0.00 | 0.07bef | 0.00 | 0.09cd | 0.00 | 0.10cd | 0.01 |
| T-BMD/kg wt (mg/cm2/kg) | 0.06f | 0.01 | 0.07f | 0.01 | 0.05ef | 0.00 | 0.05ef | 0.00 | 0.08cd | 0.00 | 0.10abcd | 0.01 |
| F-breaking strength (kg*f) | 6.89 | 0.34 | 7.39c | 0.26 | 5.69bdef | 0.15 | 7.22c | 0.26 | 7.76c | 0.40 | 7.58c | 0.58 |
| T-breaking strength (kg*f) | 4.83bd | 0.30 | 6.10ac | 0.20 | 4.11bdef | 0.14 | 6.14ac | 0.25 | 5.24c | 0.22 | 5.79c | 0.26 |
Bone metabolism and serum insulin concentration (Table 3)
Figure 2.
Bone mineral density (BMD) and bBreaking strength of femur and tibia Values are means ± SE for per groups. (A) = femur BMD; (B) = tTibia BMD; (C) = femur breaking strength; (D) = tTibia breaking strength. a p <.05 vs S, b p <.05 vs SE, c p <.05 vs O, d p <.05 vs OE, e p <.05 vs OE2, f p <.05 vs OEE. S, sham-operated and sedentary; SE, sham-operated and exercise trained; O, ovariectomized and sedentary; OE, ovariectomized and exercise trained; OE2, ovariectomized and given 17β-estradiol; OEE, ovariectomized, exercise trained, and given 17β-estradiol.

Figure 3.
Micro-computed tomographyCT of the proximal tibia Values are means ± SE for per groups. (A)= BV/TV; (B)B= TB.Th; (C)= Tb.N; (D)= Tb.Sp. a p <.05 vs S, b p <.05 vs SE, c p <.05 vs O, d p <.05 vs OE, e p <.05 vs OE2, f p <.05 vs OEE. S, sham-operated and sedentary; SE, sham-operated and exercise trained; O, ovariectomized and sedentary; OE, ovariectomized and exercise trained; OE2, ovariectomized and given 17β-estradiol; OEE, ovariectomized, exercise trained, and given 17β-estradiol; BV/TV bone volume/tissue volume; Tb.Th, trabecular thickness; Tb.N, trabecular number; Tb.Sp, trabecular separation.
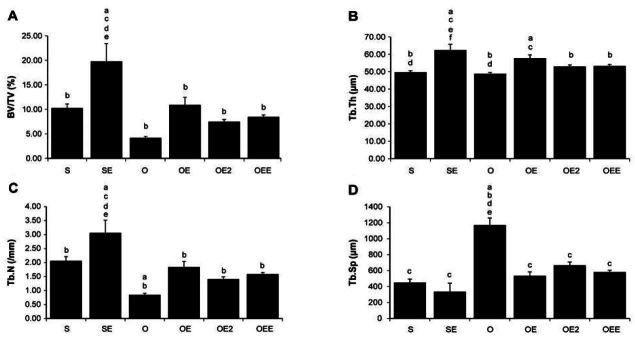
Table 3.
Biochemical markers of bone metabolism and insulin levels
|
S n=6 |
SE n=6 |
O n=10 |
OE n=11 |
OE2 n=6 |
OEE n=6 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Gla-osteocalcin | 39.08ef | 6.80 | 37.18cf | 1.69 | 52.11bef | 1.78 | 47.39ef | 3.43 | 21.27acd | 2.73 | 15.79abcd | 0.86 |
| Glu-osteocalcin | 57.71cd | 7.69 | 61.99cd | 9.77 | 116.57abef | 6.00 | 121.52abef | 12.30 | 28.94cd | 3.73 | 26.29cd | 2.88 |
| Gla/Glu-osteocalcin | 0.79 | 0.22 | 0.68 | 0.12 | 0.45 | 0.02 | 0.41 | 0.04 | 0.66 | 0.06 | 0.64 | 0.08 |
| Insulin (μg/L) | 1.14 | 0.32 | 0.55 | 0.18 | 3.26 | 1.46 | 1.41 | 0.21 | 2.27 | 0.50 | 1.87 | 0.61 |
Tibia cancellous bone parameters as revealed by Micro-CT(Table 4)
Table 4.
Trabecular parameters of the proximal tibia cancellous bone by micro-computed tomography
|
S n=6 |
SE n=6 |
O n=10 |
OE n=11 |
OE2 n=6 |
OEE n=6 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| BV/TV (%) | 10.26b | 0.84 | 19.77acdef | 3.66 | 4.18bd | 0.29 | 10.92bc | 1.55 | 7.48b | 0.45 | 8.46b | 0.39 |
| Tb.Th (μm) | 49.66bd | 0.97 | 62.44acef | 3.38 | 48.81bd | 0.75 | 57.76ac | 1.87 | 52.99b | 0.91 | 53.27b | 0.83 |
| Tb.N (/mm) | 2.06bc | 0.15 | 3.06acdef | 0.45 | 0.85abd | 0.05 | 1.84bc | 0.20 | 1.41b | 0.08 | 1.59b | 0.06 |
| Tb.Sp (μm) | 450.74c | 42.47 | 336.18c | 105.49 | 1170.49abdef | 89.32 | 535.20c | 49.38 | 666.95c | 40.82 | 581.38c | 24.23 |
| TBPf (/mm) | 12.55bcdef | 0.07 | 7.70a | 0.78 | 7.99a | 0.64 | 6.27a | 0.50 | 5.08a | 0.70 | 5.11a | 0.72 |








