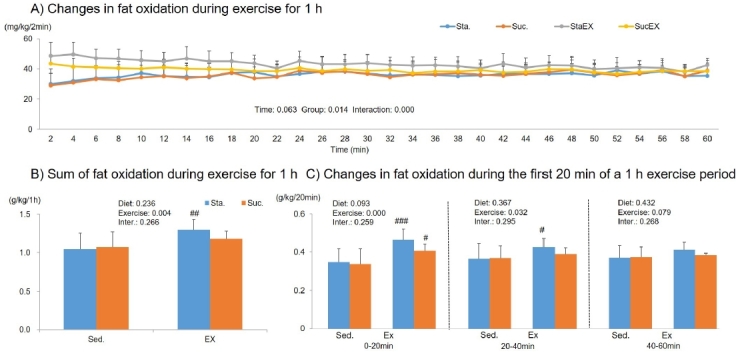
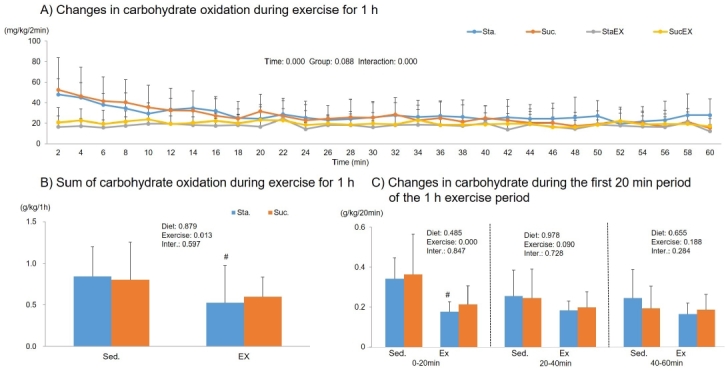
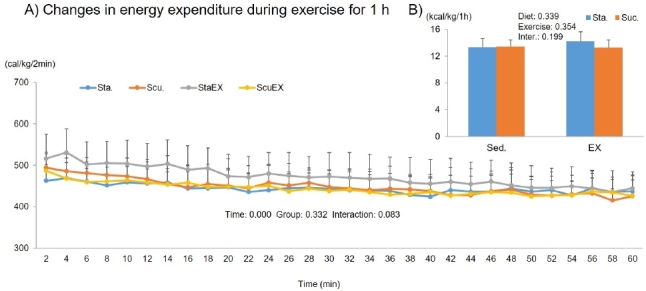
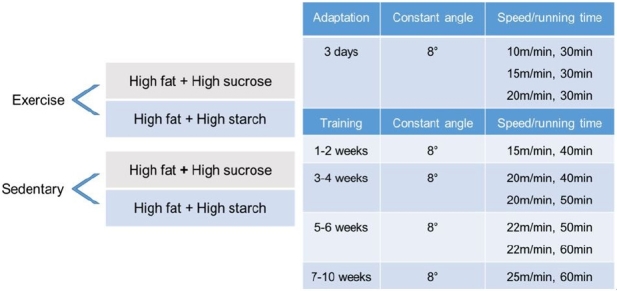
1.
Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O'Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century.
Am J Clin Nutr. 2005;81:341-54.


Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O'Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century.
Am J Clin Nutr. 2005;81:341-54. PMID:
10.1093/ajcn.81.2.341. PMID:
15699220.
2.
Ministry of Culture, Sports and Tourism. Survey on national leisure activities 2016-2018. MCST. 2019.
. Ministry of Culture, Sports and Tourism. Survey on national leisure activities 2016-2018. MCST. 2019.
3.
Popkin BM. Nutrition transition and the global diabetes epidemic.
Curr Diab Rep. 2015;15:64.


. Popkin BM. Nutrition transition and the global diabetes epidemic.
Curr Diab Rep. 2015;15:64PMID:
10.1007/s11892-015-0631-4. PMID:
26209940.
4.
Schwartz RS, Ravussin E, Massari M, O’Connell M, Robbins DC. The thermic effect of carbohydrate versus fat feeding in man.
Metabolism. 1985;34:285-93.

Schwartz RS, Ravussin E, Massari M, O’Connell M, Robbins DC. The thermic effect of carbohydrate versus fat feeding in man.
Metabolism. 1985;34:285-93. PMID:
10.1016/0026-0495(85)90014-9. PMID:
3883098.
5.
Hermsdorff HH, Volp AC, Bressan J. Macronutrient profile affects diet-induced thermogenesis and energy intake. Arch Latinoam Nutr. 2007;57:33-42.
Hermsdorff HH, Volp AC, Bressan J. Macronutrient profile affects diet-induced thermogenesis and energy intake.
Arch Latinoam Nutr. 2007;57:33-42. PMID:
17824197.
6.
Flatt JP. Dietary fat, carbohydrate balance, and weight maintenance: effects of exercise.
Am J Clin Nutr. 1987;45:296-306.


Flatt JP. Dietary fat, carbohydrate balance, and weight maintenance: effects of exercise.
Am J Clin Nutr. 1987;45:296-306. PMID:
10.1093/ajcn/45.1.296. PMID:
3799520.
7.
Kanarek RB, Orthen-Gambill N. Differential effects of sucrose, fructose and glucose on carbohydrate-induced obesity in rats.
J Nutr. 1982;112:1546-54.


Kanarek RB, Orthen-Gambill N. Differential effects of sucrose, fructose and glucose on carbohydrate-induced obesity in rats.
J Nutr. 1982;112:1546-54. PMID:
10.1093/jn/112.8.1546. PMID:
7047694.
8.
Granneman JG, Wade GN. Effect of sucrose overfeeding on brown adipose tissue lipogenesis and lipoprotein lipase activity in rats.
Metabolism. 1983;32:202-7.

Granneman JG, Wade GN. Effect of sucrose overfeeding on brown adipose tissue lipogenesis and lipoprotein lipase activity in rats.
Metabolism. 1983;32:202-7. PMID:
10.1016/0026-0495(83)90230-5. PMID:
6827991.
9.
Bukowiecki LJ, Lupien J, Folléa N, Jahjah L. Effects of sucrose, caffeine, and cola beverages on obesity, cold resistance, and adipose tissue cellularity.
Am J Physiol. 1983;244:R500-7.

Bukowiecki LJ, Lupien J, Folléa N, Jahjah L. Effects of sucrose, caffeine, and cola beverages on obesity, cold resistance, and adipose tissue cellularity.
Am J Physiol. 1983;244:R500-7. PMID:
10.1152/ajpregu.1983.244.4.r500. PMID:
6837766.
10.
Giusti V, Schneiter P, Thiébaud D, Landry M, Burckhardt P, Jéquier E, Tappy L. Influences of body weight, body composition, and substrate oxidation rate on resting postabsorptive glucose production and gluconeogenesis. Int J Obes Relat Metab Disord. 1996;20:842-7.
Giusti V, Schneiter P, Thiébaud D, Landry M, Burckhardt P, Jéquier E, Tappy L. Influences of body weight, body composition, and substrate oxidation rate on resting postabsorptive glucose production and gluconeogenesis.
Int J Obes Relat Metab Disord. 1996;20:842-7. PMID:
8880352.
11.
Aller EE, Abete I, Astrup A, Martinez JA, van Baak MA. Starches, Sugars and Obesity.
Nutrients. 2011;3:341-69.

Aller EE, Abete I, Astrup A, Martinez JA, van Baak MA. Starches, Sugars and Obesity.
Nutrients. 2011;3:341-69. PMID:
10.3390/nu3030341. PMID:
22254101.
12.
Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002.
Am J Clin Nutr. 2002;76:5-56.


Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002.
Am J Clin Nutr. 2002;76:5-56. PMID:
10.1093/ajcn/76.1.5. PMID:
12081815.
13.
Gyllenhammer LE, Weigensberg MJ, Spruijt-Metz D, Allayee H, Goran MI, Davis JN. Modifying Influence of Dietary Sugar in the Relationship Between Cortisol and Visceral Adipose Tissue in Minority Youth.
Obesity (Silver Spring). 2014;22:474-81.

Gyllenhammer LE, Weigensberg MJ, Spruijt-Metz D, Allayee H, Goran MI, Davis JN. Modifying Influence of Dietary Sugar in the Relationship Between Cortisol and Visceral Adipose Tissue in Minority Youth.
Obesity (Silver Spring). 2014;22:474-81. PMID:
10.1002/oby.20594. PMID:
23929660.
14.
Jenkins AL, Morgan LM, Bishop J, Jovanovski E, Vuksan V. Randomized Clinical Trialin Healthy Individualson the Effect of Viscous Fiber Blend on Glucose Tolerance When Incorporated in Capsules or into the Carbohydrate or Fat Component of the Meal.
J Am Coll Nutr. 2014;33:400-5.

Jenkins AL, Morgan LM, Bishop J, Jovanovski E, Vuksan V. Randomized Clinical Trialin Healthy Individualson the Effect of Viscous Fiber Blend on Glucose Tolerance When Incorporated in Capsules or into the Carbohydrate or Fat Component of the Meal.
J Am Coll Nutr. 2014;33:400-5. PMID:
10.1080/07315724.2014.905762. PMID:
25303029.
15.
Kondo K, Ishikado A, Morino K, Nishio Y, Ugi S, Kajiwara S, Kurihara M, Iwakawa H, Nakao K, Uesaki S, Shigeta Y, Imanaka H, Yoshizaki T, Sekine O, Makino T, Maegawa H, King GL, Kashiwagi A. A high-fiber, low-fat diet improves period on disease markers in high-risk subjects: apilotstudy.
Nutr Res. 2014;34:491-8.

Kondo K, Ishikado A, Morino K, Nishio Y, Ugi S, Kajiwara S, Kurihara M, Iwakawa H, Nakao K, Uesaki S, Shigeta Y, Imanaka H, Yoshizaki T, Sekine O, Makino T, Maegawa H, King GL, Kashiwagi A. A high-fiber, low-fat diet improves period on disease markers in high-risk subjects: apilotstudy.
Nutr Res. 2014;34:491-8. PMID:
25026916.
16.
Breneman CB, Tucker L. Dietary fibre consumption and insulin resistance-the role of body fat and physical activity.
Br J Nutr. 2013;110:75-83..

Breneman CB, Tucker L. Dietary fibre consumption and insulin resistance-the role of body fat and physical activity.
Br J Nutr. 2013;110:75-83. PMID:
10.1017/s0007114512004953.
17.
Storlien LH, Higgins JA, Thomas TC, Brown MA, Wang HQ, Huang XF, Else PL. Diet composition and insulin action in animal models.
Br J Nutr. 2000;83:S85-90.

Storlien LH, Higgins JA, Thomas TC, Brown MA, Wang HQ, Huang XF, Else PL. Diet composition and insulin action in animal models.
Br J Nutr. 2000;83:S85-90. PMID:
10.1017/s0007114500001008. PMID:
10889797.
18.
Alharbi M, Gallagher R, Kirkness A, Sibbritt D, Tofler G. Long-term outcomes from Healthy Eating and Exercise Lifestyle Program for overweight people with heart disease and diabetes.
Eur J Cardiovasc Nurs. 2016;15:91-9.

Alharbi M, Gallagher R, Kirkness A, Sibbritt D, Tofler G. Long-term outcomes from Healthy Eating and Exercise Lifestyle Program for overweight people with heart disease and diabetes.
Eur J Cardiovasc Nurs. 2016;15:91-9. PMID:
10.1177/1474515114557222. PMID:
25344059.
19.
Fofonka A, Ribeiro JP, Casali KR, Schaan BD. Effects of vildagliptin compared with glibenclamide on glucose variability after a submaximal exercise test in patients with type 2 diabetes: study protocol for a randomized controlled trial.
Trials. 2014;15:424.


Fofonka A, Ribeiro JP, Casali KR, Schaan BD. Effects of vildagliptin compared with glibenclamide on glucose variability after a submaximal exercise test in patients with type 2 diabetes: study protocol for a randomized controlled trial.
Trials. 2014;15:424PMID:
25366037.
20.
Inouye J, Matsuura C, Li D, Castro R, Leake A. Lifestyle intervention for filipino americans at risk for diabetes.
J Community Health Nurs. 2014;31:225-37.

Inouye J, Matsuura C, Li D, Castro R, Leake A. Lifestyle intervention for filipino americans at risk for diabetes.
J Community Health Nurs. 2014;31:225-37. PMID:
10.1080/07370016.2014.926674. PMID:
25356992.
21.
Kim J, Hwang H, Park J, Yun HY, Suh H, Lim K. Silk peptide treatment can improve the exercise performance of mice.
J Int Soc Sports Nutr. 2014;11:35.

Kim J, Hwang H, Park J, Yun HY, Suh H, Lim K. Silk peptide treatment can improve the exercise performance of mice.
J Int Soc Sports Nutr. 2014;11:35PMID:
10.1186/1550-2783-11-35. PMID:
25050085.
22.
Kim J, Park J, Kim B, Lee CH, Lim K, Suh H. Effects of Different doses of Silk Peptide on Energy Metabolism During Exercise in Mice.
J Exerc Nutrition Biochem. 2017;21:21-5.

Kim J, Park J, Kim B, Lee CH, Lim K, Suh H. Effects of Different doses of Silk Peptide on Energy Metabolism During Exercise in Mice.
J Exerc Nutrition Biochem. 2017;21:21-5. PMID:
10.20463/jenb.2017.0056.
23.
Kim J, Hwang H, Yun HY, Kim B, Lee CH, Suh H, Lim K. Silk peptide intake increases fat oxidation at rest in exercised mice.
J Nutr Sci Vitamino (Tokyo)l. 2013;59:250-5.

Kim J, Hwang H, Yun HY, Kim B, Lee CH, Suh H, Lim K. Silk peptide intake increases fat oxidation at rest in exercised mice.
J Nutr Sci Vitamino (Tokyo)l. 2013;59:250-5. PMID:
10.3177/jnsv.59.250.
24.
Hwang H, Kim J, Park J, Yun H, Cheon WK, Kim B, Lee CH, Suh H, Lim K. Red ginseng treatment for two weeks promotes fat metabolism during exercise in mice.
Nutrients. 2014;6:1874-85.

Hwang H, Kim J, Park J, Yun H, Cheon WK, Kim B, Lee CH, Suh H, Lim K. Red ginseng treatment for two weeks promotes fat metabolism during exercise in mice.
Nutrients. 2014;6:1874-85. PMID:
10.3390/nu6051874. PMID:
24803098.
25.
Kim J, Jeon Y, Hwang H, Suh H, Lim K. Effects of oral caffeine and capsaicin administration on energy expenditure and energy substrates utilization in resting rats.
J Exerc Nutr Biochem. 2011;15:183-9.

Kim J, Jeon Y, Hwang H, Suh H, Lim K. Effects of oral caffeine and capsaicin administration on energy expenditure and energy substrates utilization in resting rats.
J Exerc Nutr Biochem. 2011;15:183-9.
26.
Lim K, Kim J, Jeon Y, Hwang H, Suh H. Measurement of resting metabolic rate using metabolic chamber in resting rats.
J Exerc Nutr Biochem. 2011;15:35-40.

Lim K, Kim J, Jeon Y, Hwang H, Suh H. Measurement of resting metabolic rate using metabolic chamber in resting rats.
J Exerc Nutr Biochem. 2011;15:35-40.
27.
Kim J, Park J, Kim B, Lee CH, Lim K, Suh H. Effects of Silk Peptides Administration on Fat Utilization Over a Whole Day in Mice.
J Exerc Nutr Biochem. 2016;20:53-9.

Kim J, Park J, Kim B, Lee CH, Lim K, Suh H. Effects of Silk Peptides Administration on Fat Utilization Over a Whole Day in Mice.
J Exerc Nutr Biochem. 2016;20:53-9. PMID:
10.20463/jenb.2016.0055.
28.
Jeon YR, Kim JS, Hwang HJ, Lim KW. Effects of endurance training for 4weeks on resting metabolic rate and excess post-exercise oxygen consumption in mouse.
J Exerc Nutr Biochem. 2012;16:113-22.

Jeon YR, Kim JS, Hwang HJ, Lim KW. Effects of endurance training for 4weeks on resting metabolic rate and excess post-exercise oxygen consumption in mouse.
J Exerc Nutr Biochem. 2012;16:113-22.
29.
Sakamoto E, Seino Y, Fukami A, Mizutani N, Tsunekawa S, Ishikawa K, Ogata H, Uenishi E, Kamiya H, Hamada Y, Sato H, Harada N, Toyoda Y, Miwa I, Nakamura J, Inagaki N, Oiso Y, Ozaki N. Ingestion of a moderate high-sucrose diet results in glucose intolerance with reduced liver glucokinase activity and impaired glucagon-like peptide-1 secretion.
J Diabetes Investig. 2012;3:432-40.

Sakamoto E, Seino Y, Fukami A, Mizutani N, Tsunekawa S, Ishikawa K, Ogata H, Uenishi E, Kamiya H, Hamada Y, Sato H, Harada N, Toyoda Y, Miwa I, Nakamura J, Inagaki N, Oiso Y, Ozaki N. Ingestion of a moderate high-sucrose diet results in glucose intolerance with reduced liver glucokinase activity and impaired glucagon-like peptide-1 secretion.
J Diabetes Investig. 2012;3:432-40. PMID:
10.1111/j.2040-1124.2012.00208.x.
30.
Dulloo AG, Eisa OA, Miller DS, Yudkin J. A comparative study of the effects of white sugar, unrefined sugar and starch on the efficiency of food utilization and thermogenesis.
Am J Clin Nutr. 1985;42:214-19.


Dulloo AG, Eisa OA, Miller DS, Yudkin J. A comparative study of the effects of white sugar, unrefined sugar and starch on the efficiency of food utilization and thermogenesis.
Am J Clin Nutr. 1985;42:214-19. PMID:
10.1093/ajcn/42.2.214. PMID:
4025193.
31.
Arola L, Bonet ML, Delzenne N, Duggal MS, Gómez-Candela C, Huyghebaert A, Laville M, Lingström P, Livingstone B, Palou A, Picó C, Sanders T, Schaafsma G, van Baak M, van Loveren C, van Schothorst EM. Summary and general conclusions/outcomes on the role and fate of sugars in human nutrition and health.
Obes Rev. 2009;10:55-8.

Arola L, Bonet ML, Delzenne N, Duggal MS, Gómez-Candela C, Huyghebaert A, Laville M, Lingström P, Livingstone B, Palou A, Picó C, Sanders T, Schaafsma G, van Baak M, van Loveren C, van Schothorst EM. Summary and general conclusions/outcomes on the role and fate of sugars in human nutrition and health.
Obes Rev. 2009;10:55-8. PMID:
10.1111/j.1467-789x.2008.00565.x. PMID:
19207536.
32.
Van Baak MA, Astrup A. Consumption of sugars and body weight.
Obes Rev. 2009;10:9-23.

Van Baak MA, Astrup A. Consumption of sugars and body weight.
Obes Rev. 2009;10:9-23. PMID:
10.1111/j.1467-789x.2008.00561.x. PMID:
19207532.
33.
Kanarek RB, Aprille JR, Hirsch E, Gualtiere L, Brown CA. Sucrose-induced obesity: effect of diet on obesity and brown adipose tissue.
Am J Physiol. 1987;253:R158-66.

Kanarek RB, Aprille JR, Hirsch E, Gualtiere L, Brown CA. Sucrose-induced obesity: effect of diet on obesity and brown adipose tissue.
Am J Physiol. 1987;253:R158-66. PMID:
10.1152/ajpregu.1987.253.1.r158. PMID:
3605381.
34.
Thomas DE, Elliott EJ, Baur L. Low glycaemic index or low glycaemic load diets for overweight and obesity.
Cochrane Database Syst Rev. 2007;18:CD005105.

Thomas DE, Elliott EJ, Baur L. Low glycaemic index or low glycaemic load diets for overweight and obesity.
Cochrane Database Syst Rev. 2007;18:CD005105.
35.
Maekawa R, Seino Y, Ogata H, Murase M, Iida A, Hosokawa K, Joo E, Harada N, Tsunekawa S, Hamada Y, Oiso Y, Inagaki N, Hayashi Y, Arima H. Chronic high-sucrose diet increases fibroblast growth factor 21 production and energy expenditure in mice.
J Nutr Biochem. 2017;49:71-9.

Maekawa R, Seino Y, Ogata H, Murase M, Iida A, Hosokawa K, Joo E, Harada N, Tsunekawa S, Hamada Y, Oiso Y, Inagaki N, Hayashi Y, Arima H. Chronic high-sucrose diet increases fibroblast growth factor 21 production and energy expenditure in mice.
J Nutr Biochem. 2017;49:71-9. PMID:
10.1016/j.jnutbio.2017.07.010. PMID:
28886439.
36.
Raben A, Macdonald I, Astrup A. Replacement of dietary fat by sucrose or starch: effects on 14 d ad libitum energy intake, energy expenditure and body weight in formerly obese and never-obese subjects.
Int J Obes Relat Metab Disord. 1997;21:846-59.


Raben A, Macdonald I, Astrup A. Replacement of dietary fat by sucrose or starch: effects on 14 d ad libitum energy intake, energy expenditure and body weight in formerly obese and never-obese subjects.
Int J Obes Relat Metab Disord. 1997;21:846-59. PMID:
10.1038/sj.ijo.0800494. PMID:
9347402.
37.
Burchfield JG, Kebede MA, Meoli CC, Stöckli J, Whitworth PT, Wright AL, Hoffman NJ, Minard AY, Ma X, Krycer JR, Nelson ME, Tan SX, Yau B, Thomas KC, Wee NKY, Khor EC, Enriquez RF, Vissel B, Biden TJ, Baldock PA, Hoehn KL, Cantley J, Cooney GJ, James DE, Fazakerley DJ. High dietary fat and sucrose results in an extensive and time-dependent deterioration in health of multiple physiological systems in mice.
J Biol Chem. 2018;293:5731-45.

Burchfield JG, Kebede MA, Meoli CC, Stöckli J, Whitworth PT, Wright AL, Hoffman NJ, Minard AY, Ma X, Krycer JR, Nelson ME, Tan SX, Yau B, Thomas KC, Wee NKY, Khor EC, Enriquez RF, Vissel B, Biden TJ, Baldock PA, Hoehn KL, Cantley J, Cooney GJ, James DE, Fazakerley DJ. High dietary fat and sucrose results in an extensive and time-dependent deterioration in health of multiple physiological systems in mice.
J Biol Chem. 2018;293:5731-45. PMID:
10.1074/jbc.ra117.000808. PMID:
29440390.
38.
Granneman JG, Wade GN. Effect of sucrose overfeeding on brown adipose tissue lipogenesis and lipoprotein lipase activity in rats.
Metabolism. 1983;32:202-7.

Granneman JG, Wade GN. Effect of sucrose overfeeding on brown adipose tissue lipogenesis and lipoprotein lipase activity in rats.
Metabolism. 1983;32:202-7. PMID:
10.1016/0026-0495(83)90230-5. PMID:
6827991.
39.
Bukowiecki LJ, Lupien J, Folléa N, Jahjah L. Effects of sucrose, caffeine, and cola beverages on obesity, cold resistance, and adipose tissue cellularity.
Am J Physiol. 1983;244:R500-7.

Bukowiecki LJ, Lupien J, Folléa N, Jahjah L. Effects of sucrose, caffeine, and cola beverages on obesity, cold resistance, and adipose tissue cellularity.
Am J Physiol. 1983;244:R500-7. PMID:
10.1152/ajpregu.1983.244.4.r500. PMID:
6837766.
40.
Lockie SH, Heppner KM, Chaudhary N, Chabenne JR, Morgan DA, Veyrat-Durebex C, Ananthakrishnan G, Rohner-Jeanrenaud F, Drucker DJ, DiMarchi R, Rahmouni K, Oldfield BJ, Tschöp MH, Perez-Tilve D. Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like peptide-1 receptor signaling.
Diabetes. 2012;61:2753-62.

Lockie SH, Heppner KM, Chaudhary N, Chabenne JR, Morgan DA, Veyrat-Durebex C, Ananthakrishnan G, Rohner-Jeanrenaud F, Drucker DJ, DiMarchi R, Rahmouni K, Oldfield BJ, Tschöp MH, Perez-Tilve D. Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like peptide-1 receptor signaling.
Diabetes. 2012;61:2753-62. PMID:
10.2337/db11-1556. PMID:
22933116.
41.
Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F, Serrano M, Fernø J, Salvador J, Escalada J, Dieguez C, Lopez M, Frühbeck G, Nogueiras R. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK.
Diabetes. 2014;63:3346-58.

Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F, Serrano M, Fernø J, Salvador J, Escalada J, Dieguez C, Lopez M, Frühbeck G, Nogueiras R. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK.
Diabetes. 2014;63:3346-58. PMID:
10.2337/db14-0302. PMID:
24917578.
42.
Abbott WGH, Howard BV, Ruotolo G, Ravussin E. Energy expenditure in humans: effects of dietary fat and carbohydrate.
Am J Physiol. 1990;258:E347-51.

Abbott WGH, Howard BV, Ruotolo G, Ravussin E. Energy expenditure in humans: effects of dietary fat and carbohydrate.
Am J Physiol. 1990;258:E347-51. PMID:
10.1152/ajpendo.1990.258.2.e347. PMID:
2305878.
43.
Hurni M, Burnand B, Pittet PH, Jequier E. Metabolic effects of a mixed and a high-carbohydrate low-fat diet in man, measured over 24 h in a respiration chamber.
Br J Nutr. 1982;47:33-43.

Hurni M, Burnand B, Pittet PH, Jequier E. Metabolic effects of a mixed and a high-carbohydrate low-fat diet in man, measured over 24 h in a respiration chamber.
Br J Nutr. 1982;47:33-43. PMID:
10.1079/bjn19820006. PMID:
7037049.
44.
Schrauwen P, van Marken Lichtenbelt WD, Saris WH, Westerterp KR. Changes in fat oxidation in response to a high-fat diet.
Am J Clin Nutr. 1997;66:276-82.


Schrauwen P, van Marken Lichtenbelt WD, Saris WH, Westerterp KR. Changes in fat oxidation in response to a high-fat diet.
Am J Clin Nutr. 1997;66:276-82. PMID:
10.1093/ajcn/66.2.276. PMID:
9250105.
45.
Blaak EE, Saris WHM. Health aspects of various digestible carbohydrates.
Nutr Res. 1995;15:1547-73.

Blaak EE, Saris WHM. Health aspects of various digestible carbohydrates.
Nutr Res. 1995;15:1547-73. PMID:
10.1016/0271-5317(95)02027-s.
46.
Laville M, Nazare JA. Diabetes, insulin resistance and sugars.
Obes Rev. 2009;10:24-33.

Laville M, Nazare JA. Diabetes, insulin resistance and sugars.
Obes Rev. 2009;10:24-33. PMID:
10.1111/j.1467-789x.2008.00562.x. PMID:
19207533.